Table 3. Tryptamine Investigation for Phosphoric Acid-catalyzed Spirocyclization.
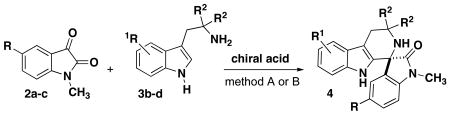 | ||||||||
|---|---|---|---|---|---|---|---|---|
| entry | product | R | R1 | R2 | method | chiral acid | yield %[a] | er[b] |
| 1 | 4bb | Cl | 6-OCH3 | H | A | 8b | 99 | 60:40 |
| 2 | 4bb | Cl | 6-OCH3 | H | B | 8d | 39 | 80:20 |
| 3 | 4bc | Cl | H | H | A | 8b | 93 | 67:33 |
| 4 | 4bc | Cl | H | H | B | 8d | 89 | 65:35 |
| 5 | 4cb | H | H | H | A | 8b | 35 | 95:5 |
| 6 | 4cb | H | H | H | B | 8d | 71 | 95:5 |
| 7 | 4bd | Cl | H | CO2CH3 | A | 8b | no rxn | -- |
| 8 | 4ad | Br | H | CO2CH3 | B | 8d | 21 | 52:48 |
| 9 | 4ad | Br | H | CO2CH3 | A | 8e | 92 | 59:41 |
Method A: 20 mol % catalyst in CH2Cl2 at 23°C for 72-96h.
Method B: 10 mol % catalyst in DMF at 40°C for 24-48h.
Isolated yield.
Enantioselectivity determined by chiral phase HPLC.
