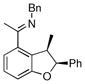
Table 1.
Enantioselective annulation of aryl imines
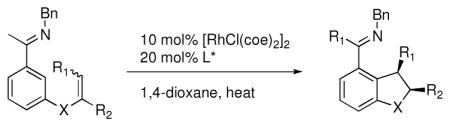
| ||||||
|---|---|---|---|---|---|---|
| Entry | Substrate | Product | L* | T (°C) | % Yield | % ee |
| 1 |
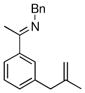
|
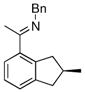
|
17b | 50 | 94 | 95 |
| 2 |
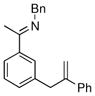
|
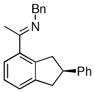
|
17c | 75 | 98 | 90 |
| 3 |
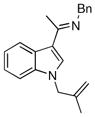
|
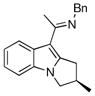
|
17c | 125 | 99 | 68 |
| 4 |
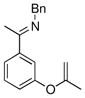
|
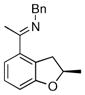
|
17c | 50 | 99 | 95 |
| 5 |
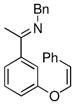
|
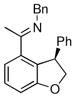
|
18a | 75 | 93 | 87 |
| 6 |
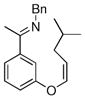
|
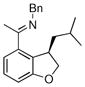
|
18a | 75 | 69 | 90 |
| 7 |
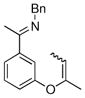
|
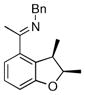
|
18b | 50 | 80 | 93 |
| 8 |
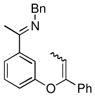
|
|
18b | 75 | 50 | 90 |
Yield of N-benzylimine product determined by 1H NMR using 2,6-dimethoxytoluene as an internal standard.
Ee’s determined after hydrolysis of products with silica gel or HCl/H2O-dioxane using chiral HPLC.
