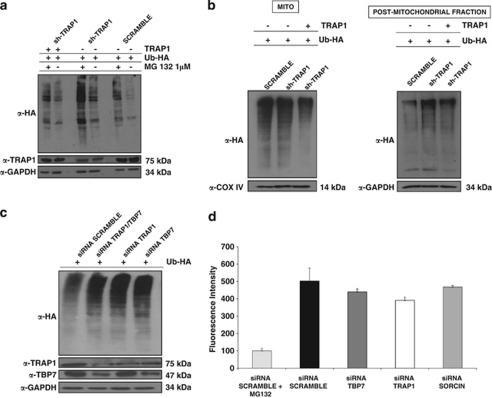
Figure 3.
Ub levels in HCT116 cells. (a) Total cell lysates from sh-TRAP1 and scrambled HCT116 stable clones were transfected with either an HA-tagged Ub vector (Ub-HA) or with TRAP1 expression vectors; treated with 1 μM MG132 for 24 h; harvested 48 h after transfection; and subjected to immunoblot using rabbit polyclonal anti-HA antibodies. The same filter was re-probed using mouse monoclonal anti-GAPDH antibodies for normalization of cell lysates. Three independent experiments were performed, with similar results. (b) Sub-cellular fractionation was obtained from sh-TRAP1 and scrambled HCT116 stable transfectants treated as described in panel a. The extracts from the PM fraction (microsomes+CYTO fraction) and mitochondria (MITO, see Materials and Methods) were separated by SDS-PAGE and immunoblotted using a rabbit polyclonal anti-HA antibody to detect Ub levels. The purity of fractions was verified by using mouse monoclonal anti-COX IV or mouse monoclonal anti-GAPDH antibodies. Three independent experiments were performed, with similar results. (c) HCT116 cells were co-transfected with a Ub-HA vector and an siRNA negative control (scramble), or with siRNAs specific for TRAP1, TBP7, or both (as indicated) and total cell lysates were harvested after 48 h from transfection. Total lysates were subjected to SDS-PAGE and immunoblotted using rabbit polyclonal anti-HA antibodies to detect total Ub levels. The same filter was re-probed using mouse monoclonal anti-GAPDH antibodies for normalization of cell lysates, and using mouse monoclonal anti-TRAP1 and mouse monoclonal anti-TBP7 antibodies. (d) Proteasome activity is not affected by TRAP1 and TBP7 silencing. Total cellular extracts were prepared after 48 h of transfection with specific siRNA for TRAP1, TBP7 or Sorcin, as control, or with an siRNA negative control (scramble), and incubated in the presence of assay buffer and the fluorogenic substrate Suc-LLVY-AMC, as described under Materials and Methods. Samples were analyzed in triplicate using an excitation wavelength of 360 nm and an emission wavelength of 450 nm to detect chymotryptic proteasome activity. The data represent the mean of three independent experiments