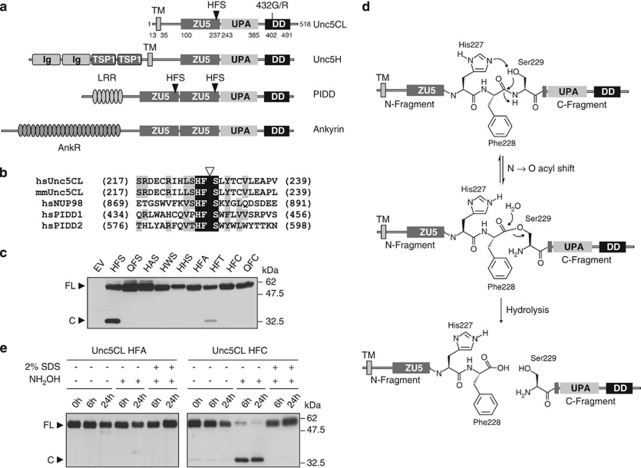
Figure 1.
Domain organization and autoproteolytic processing of Unc5CL. (a) Domain organization of ZU5-UPA-DD-containing proteins. (b) Multiple sequence alignment of the protein sequences of human (hs) and murine (mm) Unc5CL surrounding the putative autoproteolytic HFS site with the corresponding sequences from Nup98 and PIDD. Triangle: specific site of cleavage; black shading: identical amino acids; gray shading: similar amino acids. (c) HEK293T cells were transfected with the indicated C-terminally FLAG-tagged Unc5CL point mutants. FLAG-tagged proteins were analyzed by western blot. (d) Autoproteolytic cleavage mechanism at HFS sites. For autoproteolysis at the HFS site a three-step mechanism was proposed. First the histidine (His227) is involved in deprotonation of the hydroxyl group of the serine residue (Ser229). The serine hydroxyl group then functions as a nucleophile on the preceding amide bond, leading to an N → O acyl shift. The generated ester is then hydrolyzed, leading to cleavage between the phenylalanine and the serine residues. (e) HEK293T cells were transfected with the indicated C-terminally FLAG-tagged Unc5CL variants. FLAG-tagged proteins were immunoprecipitated, eluted and incubated or not with 200 mM hydroxylamine (NH2OH) ±2% SDS as described for the indicated time points. Proteins were analyzed by western blot. (c and e) FL, Unc5CL full-length protein; C, Unc5CL C-terminal cleavage fragment