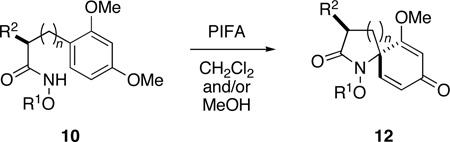
Table 1.
Preparation of Dienone Substrates 12 through Spirocyclization of Alkyl Hydroxamates 10.a
 | |||||||
|---|---|---|---|---|---|---|---|
| entry | substrate | n | R1 | R2 | product | yield (%)b | drc |
| 1 | 10a | 0 | Me | H | 12a | 53d | - |
| 2 | 10a | 0 | Me | H | 12a | 67e | - |
| 3 | 10b | 0 | Bn | H | 12b | 86e | - |
| 4 | 10c | 1 | Me | H | 12c | 81 | - |
| 5 | 10d | 1 | Bn | H | 12d | 98 | - |
| 6 | 10e | 2 | Me | H | 12e | 92 | - |
| 7 | 10f | 2 | Bn | H | 12f | 83 | - |
| 8 | 10g | 1 | Me | Me | 12g | 85f | 92:8 |
| 9 | 10h | 1 | Me | t-Bu | 12h | 82f | 87:13 |
| 10 | 10i | 1 | Me | Bn | 12i | 98g | 92:8 |
| 11 | 10j | 1 | Me | Ph | 12j | 85h | 91:9 |
| 12 | 10k | 1 | Me | OTIPS | 12k | 99i | 90:10 |
| 13 | 10l | 1 | Me | NHBz | 12l | 85f | 91:9 |
| 14 | 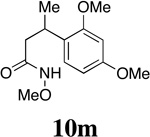 |
- | - | - | 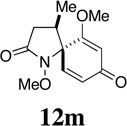 |
90f | 80:20 |
| 15 | 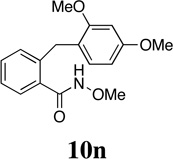 |
- | - | - | 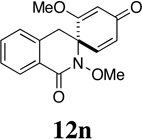 |
94 | - |
Reaction conditions: PIFA (1.2 equiv), CH2Cl2-MeOH (1:1), −78→−15 °C, 1.5 h; H2O, 10 min.
Isolated yield, after purification by flash chromatography.
Diastereomeric ratio (dr) determined by NMR analysis of the appropriate, characteristic proton signals in the unpurified product mixture.
Methanol adduct 20 (26%) was also isolated.31
Reaction performed in the absence of MeOH.
Isolated yield of anti diastereomer, after separation by flash chromatography and recrystallization.
Isolated yield of inseparable 92:8 mixture of anti and syn diastereomers.
Isolated yield of an inseparable 93:7 mixture of anti and syn diastereomers.
Isolated yield of an inseparable 90:10 mixture of anti and syn diastereomers.
