Abstract
A variety of behavioral instruments are available for assessing important aspects of cognition in both animals and humans and, in many cases, the same instruments can be used in both. While nonhuman primates are phylogenetically closest to humans, rodents, pigeons and other animals also offer behaviors worthy of note. Delay Discounting procedures are as useful as any in studies of impulsivity and may have utility in shedding light on processes associated with drug abuse. Specific memory tests such as Visual Paired Comparisons tasks (similar to the Fagan test of infant intelligence) can be modified to allow for assessment of different aspects of memory such as spatial memory. Use of these and other specific memory tasks can be used to directly monitor aspects of cognitive development in infant animals, particularly in nonhuman primates such as monkeys, and children and to draw inferences with respect to possible neuroanatomical substrates sub-serving their functions. Tasks for assessing working memory such as Variable Delayed Response (VDR), modified VDR and Spatial Working Memory tasks are now known to be affected in Parkinson’s disease (PD). These and other cognitive function tasks are being used in a monkey model of PD to assess the ability of anti-Parkinson’s disease therapies to ameliorate these cognitive deficits without diminishing their therapeutic effects on motor dysfunction. Similarly, in a rat model of the cognitive deficits associated with perinatal exposure to polychlorinated biphenyls (PCBs), clear parallels with children can be seen in at least two areas of executive function: cognitive flexibility and response inhibition. In the rat model, discrimination reversal tasks were utilized to assess cognitive flexibility, a function often assessed in humans using the Wisconsin Card Sorting Task. Response inhibition was assessed using performance in a Differential Reinforcement of Low Response Rates (DRL) task. As the data continue to accumulate, it becomes more clear that our attempts to adapt animal-appropriate tasks for the study of important aspects of human cognition have proven to be very fruitful.
1. Introduction
At the 2010 Annual Meeting of the Neurobehavioral Teratology Society held in Louisville, Kentucky, attendees benefitted from an excellent series of presentations as part of the Annual Behavioral Toxicology Symposium (NBTS Session 1). A brief review of work highlighting the use of an automated behavioral test battery for assessing aspects of cognitive function in both monkeys and children was presented by Merle Paule. Maria Alvarado discussed studies that focused on aspects of memory development in monkeys and humans by way of describing a type of memory that is dependent on the hippocampus: from a primarily anatomical perspective, different memory types and methods for assessing them were addressed. Jay Schneider followed with a presentation on the use of a monkey model of MPTP-induced Parkinson’s disease. Here, cognitive dysfunction, in addition to motor deficits, is an important characteristic of the model. Leonard Green described the use of on an intriguing choice paradigm as a behavioral metric for aspects of impulsivity, a functional domain that is affected by developmental exposure to polychlorinated biphenyls as described by Sue Schantz. It is hoped and anticipated that the approaches described here will be increasingly incorporated into the behavioral toxicologist’s tool kit.
2. Behavioral Toxicology
Our goal as behavioral toxicologists is to ultimately make predictions about the effects of chemical exposures on brain function/cognition in humans. In an ideal world, the data that are needed to make such predictions are obtained from laboratory animal models in well controlled experiments under known conditions of exposure and, preferably, prior to any significant human exposures. The use of appropriate species is also critical. If the species being considered does not metabolize the chemical of interest in a fashion similar to that of humans, or if it does not have the same target(s) of toxicity as humans, then it will not likely be an appropriate model. Likewise, if there is interest in understanding the effects of in utero/transplacental exposures to chemicals and the animal model being considered has vastly different placentation than humans, then the model is not likely going to provide the most relevant data. The use of ‘translatable’ endpoints also decreases the uncertainty associated with the process of interspecies extrapolation and utilization of identical endpoints is best, particularly when concordance/relevance to the human condition has been demonstrated. Utilization of specific behavioral tasks, designed to target or isolate specific cognitive functions, allows for a more direct interrogation of those functions. The bundling of such tasks into batteries provides the opportunity to efficiently monitor several specific functions in the same subjects.
2.1 The National Center for Toxicological Research (NCTR) Operant Test Battery (OTB)
For over two decades the National Center for Toxicological Research (NCTR) Operant Test Battery (OTB) has been utilized in multiple assessments of both acute and chronic drug exposures in monkeys (e.g., Paule 1990; 2001; Paule et al., 1992; Schulze et al., 1988). The NCTR OTB is an automated instrument that utilizes positively-reinforced tasks to assess aspects of motivation, color and position discrimination, learning, shortterm memory and timing ability. Subjects interact with press-plates and response levers to indicate choices in tasks governed by specific rules that are dependent upon the specific brain function to be assessed. Importantly, the very same apparatus and tasks can be used with human subjects: in animals, correct responses are reinforced with food pellets and in humans they are typically reinforced with money (nickels; e.g., Chelonis et al., 2000; 2002; 2004; 2011), although candy seems to work fine as well (Daniels-Shaw et al., 2001).
2.1.1. The Issue of Relevance
Of clear significance is the observation that several metrics of OTB performance in children correlate significantly with IQ (Paule et al., 1999). This characteristic actually allows for the generation of IQ estimates based solely upon OTB performance (Hashemi et al., 1994). In addition, the OTB performance of children given videotaped instructions about how to perform the tasks is often indistinguishable from that of well-trained rhesus monkeys (Paule et al., 1990). [The use of videotaped instructions to children typically provides the needed information about task rules to allow for immediate, near optimal performance, whereas animals typically require months of training]. These last two characteristics serve to indicate both the relevance of the instrument (OTB performance can serve as a surrogate for IQ) and its translational capabilities (the same endpoints can be obtained from both animals and humans). Importantly, where data exist for the behavioral effects of the same psychotropic drug in both monkeys and humans, there is a compelling ability of the monkey model to predict effects in humans (see Paule 2001 for a summary). An additional strength of the OTB and similar approaches lies in their usefulness in targeting specific cognitive domains and the ability to use them repeatedly in the same subject over extended periods of time (years; see for example Paule et al., 2011).
2.1.2. Use in Developmental, Pediatric and Juvenile Models
The OTB has been used in a developmental context in monkeys in several chronic drug studies including those on prenatal cocaine exposure (Chelonis et al., 2003) where, as in humans, infant monkeys exposed to cocaine were born with smaller head circumferences and overall length than controls (Morris et al., 1997). As adults, animals exposed to cocaine throughout gestation exhibited, as do humans similarly exposed, diminished behavioral plasticity (Chelonis et al., 2003). In a study of chronic (one year) marijuana smoke exposure during the peripubertal period, evidence was obtained from monkeys supporting the hypothesis that such marijuana use results in an ‘amotivational syndrome’ (Paule et al., 1992) as also reported for adolescent and young adult humans. Studies of the chronic pediatric administration of the anticonvulsant remacemide (Popke et al., 2001a and b) demonstrated clear and long-lasting deficits in learning task behavior in monkeys exposed for 18 months: recovery of function was not evident when the study ended six months after treatment was terminated. More recently, findings from studies on the long-term cognitive effects of a single episode of neonatal general anesthesia have been reported (Paule et al., 2011). In these studies it was shown in the rhesus monkey that a 24 hour period of ketamine-induced general anesthesia on day 5 or 6 of life resulted in significant cognitive deficits lasting several years, perhaps permanently. Clear deficits in learning capabilities and response speed were the hallmarks of this single neonatal exposure and these findings served as ‘proof-of-concept’ that some CNS-active drugs have the potential to seriously disrupt normal development and compromise subsequent brain function when exposures occur during critical periods of development.
3. Ontogeny of Hippocampal-Dependent Memory Processes: Developmental Amnesia in Monkeys and Humans
The neural system supporting declarative memory (memory of facts as opposed to memory of procedures) in humans is similarly important for memory in monkeys. Understanding the developmental time-course of the anatomical and functional components of this system is important not only for our understanding of the ages at which different neural systems and memory processes emerge, but also for identifying windows of vulnerability to the effects of neural insult. Although the research discussed here utilizes the lesion method and, as such, does not directly address the issue of toxicology, the behavioral tasks used, the memory processes they measure, and the neural structures that are manipulated are similar in both humans and monkeys and, thus, provide an opportunity for modeling the effects of toxic agents on cognition throughout development.
It seems logical that the earliest age at which subjects can perform a given memory task is the age at which its neural substrate is functional. By this reasoning, the medial temporal lobe appears to be functional early in life in humans and monkeys. Yet, in animals and humans, memory processes that are sensitive to hippocampal dysfunction in adults mature at different ages (see Alvarado and Bachevalier, 2000; Lavenex et al., 2007; Seress and Riback, 1995). For example, recognition memory develops early in humans and monkeys, whereas spatial or relational memory matures much later (Alvarado and Bachevalier, 2000; Bachevalier and Vargha-Khadem, 2005; Zeamer et al., 2010a). One explanation is that there may be (at least) two functionally separate circuits in the hippocampus that differentially support performance of certain memory tasks: the trisynaptic pathway and the direct, or temporoammonic pathway (e.g. Brun et al., 2002; Sybirska et al., 2000). It is perhaps the differential maturation of these two pathways that accounts for both qualitative as well as quantitative changes in “hippocampal memory” during development (see Alvarado & Bachevalier, 2000).
3.1 Differential Hippocampal Development
Humans, monkeys and rodents undergo considerable postnatal changes in hippocampal structure and function. In monkeys, hippocampal neurogenesis is largely complete at, or shortly after, birth (Seress, 2007; Alvarado & Bachevalier, 2000; Lavenex 2007) and the direct temporoammonic pathway appears to be present at birth. Conversely, maturation of the trisynaptic pathway is quite protracted. Neurogenesis in the dentate gyrus, although 80% complete at birth, continues throughout the first several months of life and migration and maturation of dentate cells continues through at least the first year. CA3 pyramidal cells also undergo protracted maturation during the first year of postnatal life (Seress & Riback, 1995) with synaptogenesis and myelination continuing during this time and the overall volume of the hippocampus increasing from birth to 11 months of age (Payne et al., 2010). Although the precise timing differs, humans also show this same developmental pattern extending at least throughout the first 5 postnatal years (Seress, 2001, 2007). Thus, the two putative hippocampal networks which may differentially support memory, also develop differentially, with the direct pathway that may support recognition memory being present shortly after birth, and the trisynaptic pathway that may support spatial and relational memory developing over the first year and showing further maturation over the first 2 years.
3.2 Differential Development of Hippocampal Memory
The following sections describe ongoing experiments that may provide insight into whether the development of hippocampal-dependent memory can be explained by maturational changes in the direct vs. the trisynaptic pathways. Testing at several ages over the first 3 years of life, normal cognitive development was explored, as were the effects of neonatal removal of the hippocampus or the perirhinal cortex (major input into the direct pathway), on cognitive development. Nursery-reared infant rhesus macaques were used (see: Goursaud & Bachevalier, 2006). Some received injections of the selective neurotoxin ibotenic acid into the hippocampal formation or the perirhinal cortex at 10–12 days of age (Zeamer & Bachevalier, 2009; Zeamer et al., 2010b), others served as sham surgical controls or received no intervention. Item-specific recognition, spatial recognition, or relational memory were then assessed at ages before and after the hippocampus is anatomically mature. It was expected that memory tasks that preferentially engaged the direct pathway would emerge early in life and be sensitive to both perirhinal and hippocampal damage, whereas those that engaged the trisynaptic pathway would emerge relatively late (1–2 years of age) and be sensitive to complete hippocampal damage.
3.3 Recognition Memory Development
Recognition memory was tested using the visual paired comparison (VPC) task. In this task, subjects were familiarized to a visual image, and then after a brief delay, were presented with the original image paired side-by-side with a novel image. Capitalizing on the subject’s innate preference for novelty, memory is inferred from the subject’s preference for looking at the novel image. VPC requires no training and can be administered to young infants (Fagan, 1970) including humans. It is particularly sensitive to hippocampal damage in adult monkeys (Nemanic et al., 2004; McKee & Squire, 1993; Zola, et al., 2000), which makes it an ideal candidate for assessing early memory development. Recognition memory was assessed at 1.5, 6 and 18 months of age using retention spans of 10, 30, 60 and 120 seconds (Zeamer et al., 2010b).
At 1.5 months of age, control infant monkeys showed a clear novelty preference of about 65% across all 4 delays (reliably different from chance). At 6 months, their novelty preference was stronger, averaging 73% across all delays. However, at 18 months a different pattern emerged: the novelty preference of control monkeys showed a delay-dependent decline, with novelty preference being weaker at 120 sec (65%) than at 10 sec (75%), although still reliably different from chance. These data suggest that qualitative changes are occurring in hippocampal function between 6 and 18 months of life and also suggest that the neural substrate for recognition memory at 1.5 & 6 months is not identical to that at 18 months. Possibly, the early ability to perform the VPC task is supported by a functional direct pathway and the change in performance seen at 18 months reflects contributions from the later-maturing trisynaptic pathway. Thus, a complete hippocampal lesion that disrupts both pathways should impair performance at all ages tested.
Contrary to that prediction, infant monkeys with hippocampal damage, performed identically to controls. By contrast, at 18 months of age, this group was significantly impaired at the longest delay. Thus, in monkeys, hippocampal-dependent recognition memory develops between 6 and 18 months of age. Intact recognition memory at 1.5 and 6 months in both controls and lesioned infants can be supported by another neural substrate, rather than the direct pathway. One likely candidate is the perirhinal cortex, which, along with the entorhinal cortex, plays an important role in recognition memory (Nemanic et al., 2004; Buffalo et al., 1999). The perirhinal cortex is adult-like perinatally, and lesions to it during the neonatal period impair acquisition and performance of delayed nonmatch-to-sample tasks (Málková et al., 2001). Thus, if this area is functional early on, then neonatal damage should impair memory at 1.5 and 6 months.
Preliminary data partially support this hypothesis. Neonatal damage to the perirhinal cortex alone reduced novelty preference, as compared to controls, at all three ages, and this deficit became more severe with age such that at 18 months the perirhinal-lesioned monkeys differed from controls at all delays (Zeamer & Bachevalier, 2009; Zeamer et al., 2010b). Importantly, perirhinal lesions did not abolish reliable preference for novelty at any delay. Thus, the magnitude of impairment differs strikingly from that seen in adults (Nemanic et al., 2004; Buffalo et al., 1999). This last finding indicates that the neural network supporting recognition memory in infancy includes the perirhinal cortex, but is more widespread than in the adult (Zeamer et al., 2010b).
3.4 Spatial Memory Development
Spatial memory was assessed at 9 and 24 months of age and again at 5 years using VPC tests designed to assess spatial memory (Blue et al., 2009). Monkeys were tested on two VPC variations. 1) In the Location task, monkeys were familiarized to an image of an object located in one position on the screen. Following a brief delay, the object in the familiarized location was presented along with that same object in a different location. Monkeys should preferentially look to the new location. 2) In the object-in-place (Obj-Place) task, monkeys were familiarized to a circular array of 5 objects. Following a brief delay, the original array was presented side-by-side with a new array in which 3 of the 5 objects switched positions. Sensitivity to the spatial relationships amongst the objects should yield a preference for looking at the rearranged image. Damage to the hippocampus or temporal cortical areas in adult monkeys differentially impacts performance on these variations. In adults, hippocampal damage impairs performance on Obj-Place, but not the Location task (Bachevalier & Nemanic, 2008).
Similar results were obtained here for the infants. For the Location task, normal controls performed at chance levels at 9 months of age, but showed strong novelty preference at 24 months and 5 years of age. Early hippocampal damage impaired performance at 9 and 24 months, but novelty preference returned to normal at 5 years of age. This transient impairment on the Location task is similar to the sparing observed in adults and may reflect temporal cortical involvement (Bachevalier & Nemanic, 2008). For the Obj-Place task, normal controls performed at chance levels at 9 and 24 months of age, but showed strong novelty preference at 5 years of age. Unlike the effect on the Location task, neonatal hippocampal damage permanently impaired performance on Obj-Place. Thus, as measured by performance of a VPC task, location recognition memory emerges much later than item-specific recognition memory, but can be subserved by temporal cortical structures when they have matured (Bachevalier & Nemanic, 2008; Blue et al, 2009). By contrast, spatial relational memory develops after 2 years of age, is fully mature by 5 years and requires the fully functional hippocampus for performance. Monkeys with early perirhinal damage are currently being assessed, but to the degree that perirhinal cortex contributes to memory for objects at any age, impairment is expected on the Obj-Place task, but not necessarily in the Location task.
3.5 Relational Memory Development
Since it has been shown that spatial relational memory emerges after 24 months of age and requires the hippocampus, the next logical question was whether non-spatial relational memory abilities appear at an age consistent with maturation of the trisynaptic pathway, and whether neonatal hippocampal damage effectively prevents the development of relational memory. To assess the normal development of non-spatial relational memory, monkeys were trained to perform a transverse patterning task, which has been shown to be sensitive to hippocampal damage in monkeys (Alvarado & Bachevalier, 2005) and rodents (Alvarado & Rudy, 1995) and that is late-developing in humans (Rudy et al., 1993). In this task, subjects are required to solve three, concurrent discrimination problems formed from only 3 stimuli, A, B and C. The discrimination problems are formed such that each stimulus appears in two discriminations, and is reinforced in one, but not the other as follows: 1) A+ vs. B−; 2) B+ vs. C−; 3) C+ vs. A− (+ indicates reinforced, − indicates not reinforced). To perform well on this task, subjects must disregard the reinforcer contingencies for the stimuli (50%) and attend to the relationship or conjunction of stimuli to guide correct responding; that is, “if A and B are present, choose A” (Alvarado & Rudy, 1992).
Macaques were trained at 3–4 months, 6 months, 1, 2 or 3 years of age. Each subject was trained at one age to a criterion of 90% correct performance averaged across the three discriminations and a minimum of 80% correct on any one problem. We found that, whereas performance gradually improved with age from chance at 3–4 months, it was only the 3-year-olds who performed in an adult-like way (see Alvarado & Bachevalier, 2000 for preliminary data). Monkeys at 12 and 24 months of age were able to perform well on two of the discriminations, but failed the third, indicating that they were using item-based rather than relational strategies (Alvarado & Bachevalier, 2005). Interestingly, when monkeys who failed to learn at the younger ages were retested at either 1, 2 or 3 years of age, successful performance was observed in animals as young as 12 months and was present in all juveniles re-tested at 2 years and older. This suggests that the neural architecture supporting relational learning is available between 1–2 years of age.
3.6 Comparison with Human Memory Development
It is important to note that human infants exhibit a similar developmental profile. That is, recognition memory as measured by performance of the VPC task is present early on and improves with age (see Richmond & Nelson, 2007), but relational memory abilities develop around 5 years of age, as shown by tasks such as transverse patterning (Rudy et al., 1993) and spatial navigation (Overman et al., 1996). Similar to findings reported here, patients with developmental amnesia, who sustained selective hippocampal damage in infancy or childhood, were impaired on the VPC task when tested as adults (Muñoz et al., 2010). They were also impaired on spatial and relational memory tasks (Spiers et al., 2001; Kumaran et al., 2007). Although developmental amnesic patients have not been tested specifically on transverse patterning, adult amnesic patients are quite impaired on this task (Rickard et al., 2006).
In sum, cognitive processes that are sensitive to hippocampal dysfunction show protracted development in monkeys, as in humans. Although the nature of an early-developing direct pathway is still in question, it is clear that the full trisynaptic pathway matures over the first 12–18 months in monkeys and over the first 5–7 years in humans. The neural network supporting early memory neither requires the direct pathway, nor is identical to that in the adult. Additional studies in monkeys will be particularly useful in determining which circuits are important in infancy and the long-term consequences of damage to those systems. The evidence that developmentally amnesic children or adults with hippocampal damage are similarly impaired on these cognitive tasks makes these tasks useful comparative measures for drug effects (e.g. Gunderson et al, 1988; Oken et al., 2005) on human and monkey cognition.
4. Modeling the Cognitive Deficits of Parkinson’s Disease
Parkinson’s disease (PD) is most distinctly a disorder of motor function, yet PD patients often present with a variety of non-motor features that are potentially major sources of disability (Olanow et al., 2009). Among these, cognitive impairment is a common feature, even in the early stages of the disease (Brown and Marsden, 1990; Cooper et al., 1991; Hietanen and Teravainen, 1986; Levin et al., 1989; Owen et al., 1992; Sawamoto et al, 2002). Cognitive impairment in PD is present in patients without dementia or psychosis, but may serve as a predictor of later development of dementia (Verbaan et al, 2007). The cognitive impairments seen in PD often involve executive function, attention, visuospatial performance and working memory, suggesting a frontal or fronto-striatal nature to these cognitive deficits. These deficits can include: difficulty in attention set shifting and set formation (Downes et al, 1989; Flowers et al, 1985), temporal ordering, sequencing, and planning (Cooper et al, 1991); impaired short-term recall; impaired short-term memory (Freedman et al, 1986); and impairments in attention (Sharpe et al, 1990, 1992). Although there are no universal approaches for identifying cognitive impairment in PD, recent studies suggest that up to ~50% of treated PD patients may exhibit cognitive dysfunction (or ‘mild cognitive impairment’) (Fernandez, 2005; Mamikonyan et al., 2009; Zalonis et al, 2010) without frank dementia (i.e., normal Mini Mental State Exam score).
4.1 Treating PD-Associated Cognitive Deficits
Ameliorating the cognitive deficits associated with PD has proven to be difficult. While dopamine (DA) replacement with levodopa (L-dopa) remains the gold standard for the relief of the motor symptoms of PD, this drug has had inconsistent effects on cognition and may even worsen at least some aspects of cognitive functioning in some PD patients (Cools et al., 2001). Thus, remediation of the cognitive deficits associated with PD remains a major unmet medical need of this patient population. In particular, therapies are needed that can be administered adjunctively with L-dopa to improve cognition (or counteract potential detrimental effects of L-dopa on cognition) and not diminish the therapeutic effects of L-dopa on motor functioning. Unfortunately, such drug development efforts have been hampered in part due to lack of an appropriate animal model in which to conduct preclinical studies.
4.2 Nonhuman Model of PD and Cognitive Assessments
In order to fill this void, a nonhuman primate (rhesus monkey) model of Parkinsonism was developed that involves the administration of low doses of the dopaminergic neurotoxin 1-methyl, 4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) over extended periods of time [i.e., chronic low dose (CLD) MPTP administration]. This model was initially developed to produce cognitive impairments similar to those seen in PD patients but without the debilitating motor impairments and the intent of initial studies discussed here was to examine cognitive dysfunction associated with the early stages of PD. CLD MPTP animals developed a number of ‘frontal’ cognitive deficits analogous to those described in early PD patients but in the absence of distinct Parkinsonian motor deficits. In these “early” CLD MPTP monkeys, PD-relevant cognitive functions such as spatial and non-spatial working memory, short-term and reference memory, sustained and selective attention, and aspects of executive functioning were studied. Deficits in performance of delayed response, variable delayed response, delayed alternation, delayed matching-to-sample, object retrieval, discrimination reversal, attention (set) shifting, and a continuous performance task have been documented (Schneider and Kovelowski, 1990; Schneider and Roeltgen, 1993; Decamp and Schneider, 2004). The use of analogous tasks designed for humans [i.e., delayed response (Postle et al., 1997), attention set shifting (Lange et al., 1992), delayed matching-to-sample (Owen et al., 1993), continuous performance (Stern et al., 1984), have also detected impairments in performance in PD patients. No problems with visual discrimination performance (reference memory) have been found in MPTP-treated monkeys or non-demented PD patients.
However, in view of the presence of the noted attentional deficits in these animals it was unclear the extent to which deficits in performance of delayed response and delayed matching-to-sample tasks were related to an attentional disturbance or a working memory impairment. Because of the nature of the tasks that were used, it was not possible to unequivocally separate attention from memory components of task performance. Therefore, we examined performance of CLD MPTP treated monkeys with stable cognitive deficits using both a standard variable delayed response (VDR) paradigm and a modified VDR task with attentional cueing (Decamp et al, 2004). On the standard VDR task performed using a Wisconsin general test apparatus, an opaque screen is lifted and cue presentation (the observable baiting of 1 of 2 food wells) occurs over a fixed 2 sec. interval. The opaque screen is then lowered for an intratrial delay (variable) and the food wells are covered with 2 identical sliding plates. The screen is then raised and the animal is presented with the 2 wells and is allowed to respond (i.e., uncover one of the 2 wells to retrieve the reward). The protocol for the VDR task with attentional cueing was identical to that described above except that the examiner insured that the monkey was attending to the cue presentation (i.e., directly observed the baiting of the well) before the opaque screen was lowered. Normal animals performed very well on short duration delay trials and exhibited a delay-dependent decrease in the number of correct responses with increasing delay lengths. After CLD MPTP administration, performance at short and medium duration delays (2, 5, and 10 sec.) was significantly worse than in normal animals. With attentional cueing, performance was significantly improved at these short and intermediate delays, suggesting a strong attentional component to the deficits observed using a standard “spatial working memory” task such as the VDR task.
4.3 Spatial Working Memory Assessments
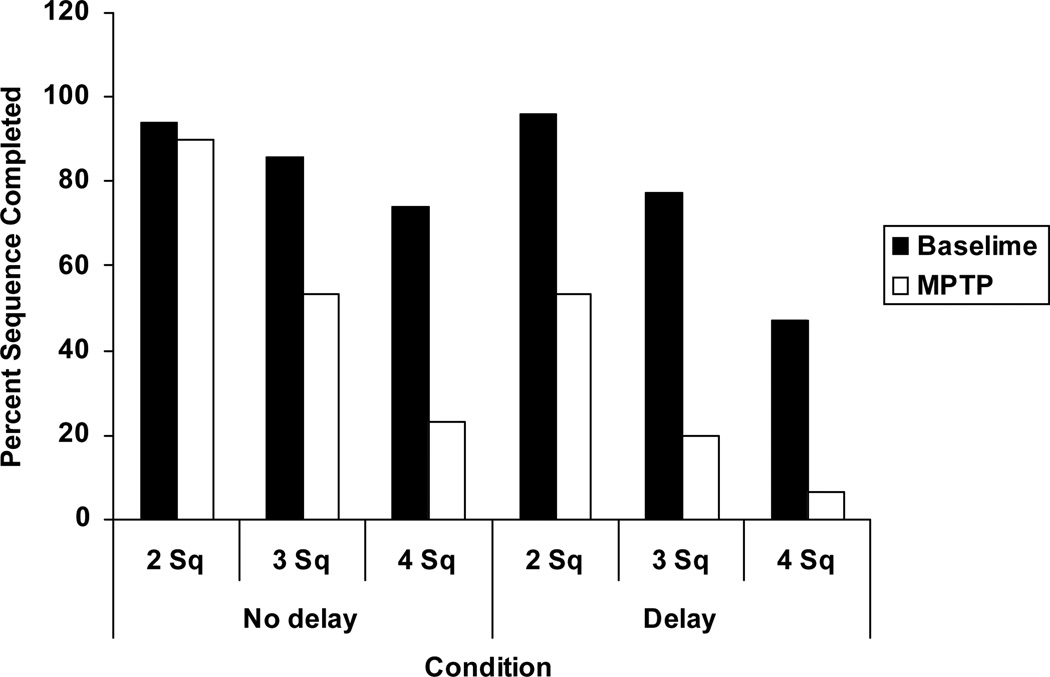
However, the question remained, do CLD MPTP monkeys have a spatial working memory (SWM) deficit as has been described in PD (and “early” PD) patients? In order to assess this, we designed a SWM task in which the level of processing and information manipulation would be higher than that in typical delayed response tasks. Briefly, the task is a self-ordered SWM task that differs from the classic delayed response (DR)-type tasks in that working memory is engaged over a much longer period than in DR tasks and memory load and level of processing necessary to perform the task are higher. These aspects of this task make it more similar to SWM tasks with executive components that have been used in human studies and which have been shown to be affected even in mild PD patients (Kulisevsky, 2000; Lewis et al, 2004; Owen et al, 1997). In this task, the subjects are presented with boxes that appear on a touch-sensitive computer monitor and they must remember the spatial locations of the boxes on the screen and select each box in turn without revisiting a box once it has been touched. The difficulty of the task can be varied by increasing the number of boxes and by introducing a delay between each touch. Using this task, CLD MPTP-treated monkeys display a SWM deficit (Figure 1). When normal, these same animals had no difficulty performing the easiest level of the task with or without a delay. At the most difficult task level, normal animals performed significantly worse in the delay condition than in the no delay condition. After CLD MPTP exposure, the same animals performed well at the easiest task level but only in the no delay condition and exhibited significant deficits at more difficult task levels under both the no delay and delay conditions. These data suggest that CLD MPTP-treated animals have a SWM deficit that was not previously apparent using the less demanding DR paradigm.
Figure 1.
Spatial working memory task performance in monkeys before and after chronic low dose MPTP exposure. In the pre-MPTP baseline condition, animals perform almost flawlessly at the easiest level of the task (2 square (Sq) sequences). As task difficulty increases, animals complete significantly less sequences correctly. Addition of a delay (5 sec) caused fewer sequences to be completed correctly, but only at medium and high levels of task difficulty. Following MPTP administration, animals developed deficits in performance at all levels of the task, but particularly at the most difficult 4 square level. Performance of these animals further worsened with addition of a delay. Baseline= normal, pre-MPTP: MPTP = post-MPTP performance.
4.4 Treatment of PD-Related Cognitive Deficits
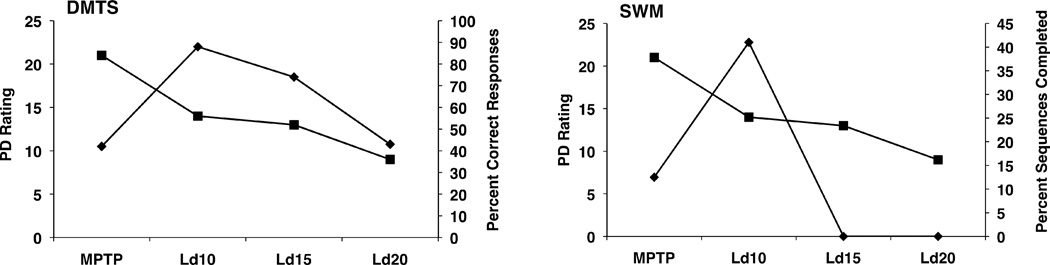
Therapies that can be administered adjunctively with L-dopa to improve cognition (or counteract potential detrimental effects of L-dopa on cognition) and not diminish the therapeutic effects of L-dopa on motor functioning are needed. As mentioned previously, the development of such therapies has been hampered in part due to lack of an appropriate animal model that recapitulates both the motor and non-motor aspects of PD (i.e., Parkinsonian motor impairment coincident with fronto-executive cognitive dysfunction). In response to this problem, an animal model has been produced by appropriately titrating chronic low dose MPTP exposure. The model exhibits cognitive and motor deficits associated with PD and which, in preliminary studies, respond to DAergic therapy in a manner similar to that which has been described in PD patients. That is, some tasks are unaffected by L-dopa while others are either improved or worsened depending on the dose of L-dopa administered. Thus far in this model, animals display moderate Parkinsonian motor deficits along with deficits in performing attention [continuous performance task (CPT)] and executive function/memory (discrimination reversal, spatial and non-spatial working memory) tasks. For example, animals previously trained to perform a delayed matching-to-sample (DMTS) task and a spatial working memory (SWM) task (described earlier), developed moderate Parkinsonian motor deficits and deficits in performing both cognitive tasks. L-dopa administration improved DMTS performance at doses that were ‘sub-optimal’ for improving motor symptoms but not at doses that were ‘optimal’ for motor improvement (Fig. 2). On the SWM task, a ‘sub-optimal’ dose of L-dopa also improved performance at both easy and difficult levels of the task, however, task performance deteriorated with increasing L-dopa dose and at a dose optimal for relieving motor symptoms, task performance was disrupted (Fig. 2). Thus, in these preliminary studies, cognitive performance on frontal-mediated tasks were improved with a dose of L-dopa sub-optimal for improving motor symptoms but worsened when a dose of L-dopa optimal for alleviating motor symptoms was administered. These findings are similar to results reported in PD patients in which some tasks are either improved or worsened depending on the dose of L-dopa administered. Such findings have been attributed to a ‘DA overdose’ condition in which administration of L-dopa may provide sufficient DA to heavily DA-denervated brain regions involved primarily with motor functioning, such as the putamen, but may ‘overdose’ less DA denervated regions, such as the pre-frontal cortex (Cools et al., 2001). This DA overdose hypothesis has been proposed to explain the variable effects of L-dopa on cognition in PD (Lewis et al, 2005; Nieoullon, 2002; Nieoullon and Coquerel, 2003; Williams and Goldman-Rakic, 1998) and now, with the availability of an appropriate animal model, it may be possible to directly assess this phenomenon and shed light on the complex issue of cognitive dysfunction and its remediation in PD.
Figure 2.
Effect of sub-optimal and optimal doses of L-dopa on motor functioning (squares) and performance on delayed matching to sample (DMTS) and spatial working memory (SWM) tasks (diamonds). L-dopa (10 mg/kg, (Ld10)) reduced motor disability by approximately 33% and significantly improved performance of both tasks. At a dose (i.e., 20 mg/kg, (Ld20)) optimal for improving motor function (i.e., approximately 57% decrease in motor disability), L-dopa failed to improve DMTS task performance and further disrupted performance of the SWM task.
5. Delay Discounting by Humans and Other Animals
When humans and other animals choose between a smaller and a larger reward, they tend to choose the larger one; when choosing between getting a reward sooner or later, they tend to choose getting the reward sooner. However, many situations call for choices between rewards that differ on more than one dimension, such as receiving either a smaller reward sooner or a larger one later. In such situations, immediacy is pitted against magnitude, and given such a choice, humans and other animals often choose the smaller reward over the larger one if the larger reward is not available until much later. Such behavior may be understood in terms of discounting – that is, the smaller, sooner reward is chosen because the value of the larger, later reward is discounted, so that its discounted value is less than the value of the smaller, sooner reward.
5.1 Rate of Discounting and Impulsivity
Steep discounting of delayed rewards raises issues of impulsivity and impatience, whereas being willing to wait for a larger reward is assumed to reflect self-control (Green and Myerson, 2010). Support for this interpretation is provided by comparisons of individuals who have been diagnosed with an impulse-control disorder, such as substance abuse. Substance abusers, including alcoholics, heroin addicts, and cigarette smokers, have been repeatedly shown to discount delayed rewards more steeply than controls (for reviews, see Bickel and Marsch, 2001; Reynolds, 2006), consistent with the idea that in humans, steep discounting reflects impulsivity. Behavioral toxicology research has shown that exposure to various substances (e.g., PCBs) increases measures of impulsivity, as discussed by Schantz in the next section, suggesting that an important consequence of such exposure may be increased risk for substance abuse. From this perspective, it may be very useful to be able to study animals using procedures directly analogous to those used to study substance abusers and other special populations (e.g., pathological gamblers). More generally discounting procedures would appear to have the potential for assessing the effects of neurotoxic substances on impulsive decision making in both humans and nonhuman animals.
5.2 Adjusting amount procedure
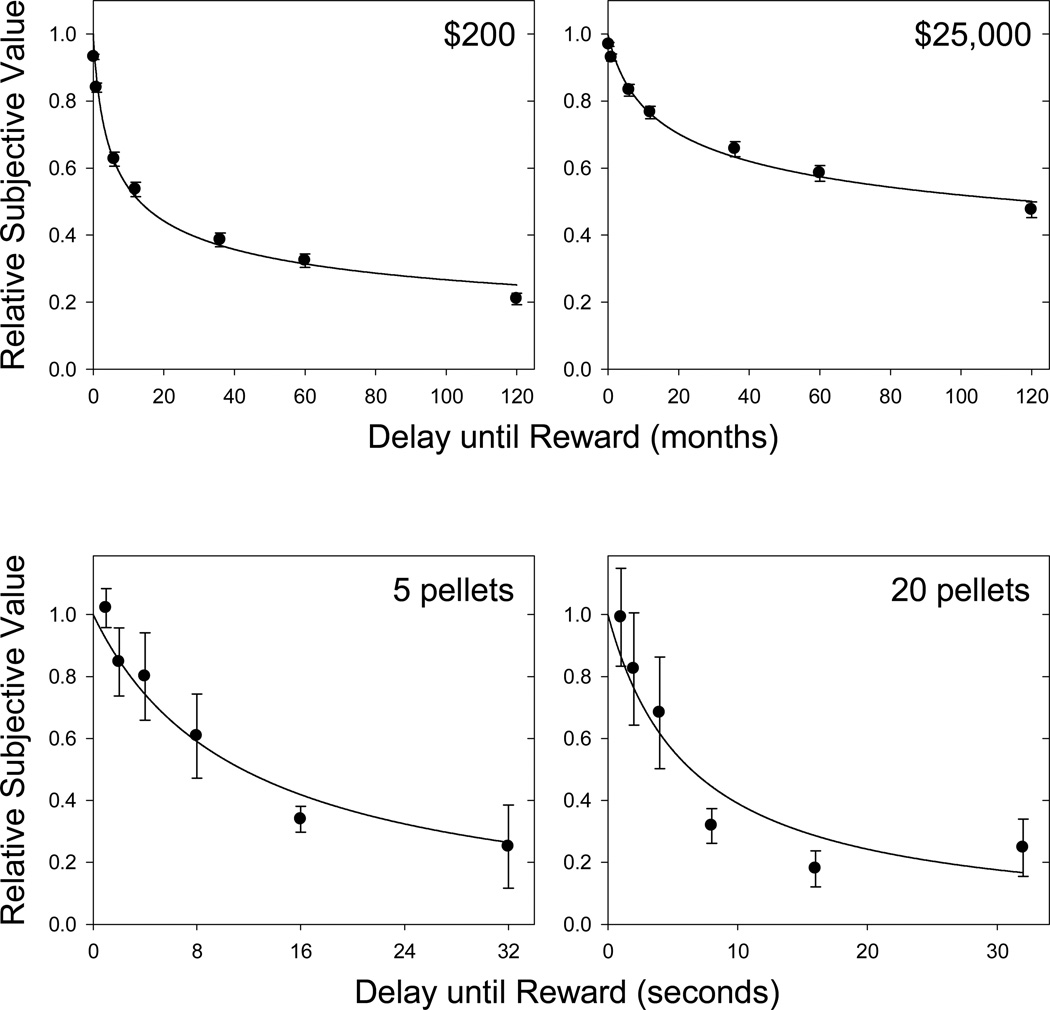
Discounting in humans has been studied principally using an adjusting-amount procedure. Participants are given a choice between a standard, fixed reward amount at a specified delay and a smaller, immediate reward whose amount is adjusted from trial to trial in order to determine the amount of immediate reward that the participant judges to be equal in value to the delayed reward. This amount of immediate reward is termed the subjective value of the delayed reward. The procedure is then repeated using different delays so as to map out a discounting function that relates the subjective value of a reward to the delay until its receipt (see the upper panels of Fig. 3). The rewards in human studies have most often been hypothetical amounts of money, and a discounting function can be mapped out in a single session.
Figure 3.
Relative subjective value of a reward as a function of the delay until its receipt. Upper panels: data from humans discounting hypothetical monetary rewards (from Myerson, Green, Hanson, Holt, & Estle, 2003); symbols and error bars represent the means and standard errors for 171 subjects. Bottom panels: data from rats discounting food pellet rewards (adapted from “Discounting of Delayed Food Rewards in Pigeons and Rats: Is There a Magnitude Effect?”, by L. Green, J. Myerson, D. D. Holt, J. R. Slevin, and S. J. Estle, Journal of the Experimental Analysis of Behavior, 81, p. 46. Copyright 2004 by the Society for the Experimental Analysis of Behavior, Inc. Reprinted with permission.); symbols and error bars represent the means and standard errors for 4 rats. The curved lines represent the fits of Equation 1 (all R2s ≥ .90); for the human data, s was a free parameter, whereas for the rat data, s was fixed at 1.0.
As may be seen in Figure 3, the data are well fit by a hyperboloid function that describes the relation between the subjective value (V) of a fixed amount (A) of reward and the delay (D) until its receipt:
| (Eq. 1) |
where s represents a nonlinear scaling parameter and k is a free parameter reflecting how steeply the delayed reward is discounted, with larger values of k representing steeper discounting. It should be noted that the curves in Figure 5 depict the fit of a hyperboloid function similar to Equation 1 except that it describes changes in the relative value of the delayed reward (i.e., the amount of immediate reward that is equal in value to the delayed reward expressed as a proportion of the delayed amount). By expressing the value of the delayed reward in relative terms, one is able to plot the data from different amounts and types of reward on the same axes in order to easily compare discounting rates.
The adjusting-amount procedure used with animals is analogous to that used with humans except that the procedure must be modified to ensure that the animals have experience with the alternatives before they are asked to choose between them. This is typically accomplished by alternating a block of forced-choice trials with a block of free-choice trials. The forced-choice trials function to inform the subject what the current alternatives are, whereas the free-choice trials serve to assess the subject’s preference. As with the procedure used with humans, the amount of the immediate reward is adjusted until the animal indicates that it finds the two alternatives to be of approximately equal value by choosing them equally often, thus indicating the subjective value of the larger, delayed reward. Once the subjective value has been determined at one delay, the procedure is repeated using the same amount of delayed reward but with different delays until its receipt, in order to map out a discounting function. Depending on the species and the exact version of the adjusting-amount procedure that is used, the process of establishing a discounting function can take anywhere from one week (Richards et al., 1997) to several months (Green et al., 2004).
5.3 Adjusting Delay Procedure
In addition to the adjusting-amount procedure, other procedures have also been used for studying discounting in animals (for a discussion of the advantages and disadvantages of different procedures, see Madden and Johnson, 2010). One method is the adjusting-delay procedure (Mazur, 1987). As with the adjusting-amount procedure, forced-choice trials alternate with free-choice trials, except that in this case, the smaller, immediate and larger, delayed reward amounts are both fixed, and what is varied is the delay until the larger reward. Importantly, the different procedures appear to produce similar results, suggesting that they all tap the same underlying behavioral processes (Green et al., 2007; Mazur, 2000).
5.4 Human to Animal Comparisons
It is now well established that the same mathematical function (Eq. 1) used to describe delay discounting by humans also describes discounting of delayed rewards by animals (see the lower panels of Fig. 3), except that with animals the value of the nonlinear scaling parameter, s, in Equation 1 is typically close to 1.0 (Calvert et al., 2010; Green et al., 2004), whereas with humans it is typically less than 1.0. Two other important differences in the discounting of delayed rewards by humans and animals may be seen in Figure 3. One is that humans discount larger delayed rewards less steeply than smaller ones (compare the two upper panels), whereas animals show equivalent discounting of larger and smaller delayed rewards (compare the two lower panels). In fact, to the best of our knowledge no study with rats or pigeons has reported shallower discounting of larger delayed rewards despite the fact that this is a robust finding in humans, at least in studies using either hypothetical or real monetary rewards (for a review, see Green and Myerson, 2004).
The other difference is that animals have been reported to discount much more steeply than humans (compare the time scales in the upper and lower panels of Fig. 3). However, this often-reported difference may be peculiar to the types of rewards typically studied with humans, rather than being a true difference between humans’ and animals’ discounting of delayed rewards: When humans discount delayed juice rewards that they actually consume following each choice, rather than hypothetical rewards such as money which are not themselves directly consumable, they discount such rewards on a time scale much more similar to that observed in animal studies (Jimura et al., 2009). Even when tested with directly consumable rewards that are hypothetical, such as candy or soda, they discount such rewards much more steeply than they do monetary rewards (Estle et al., 2007; Odum and Rainaud, 2003), although not as steeply as they do real directly consumable rewards. Taken together, these results suggest that the time scale difference in discounting by humans and animals may be more apparent than real.
5.5 Animal Models of Impulsive Behavior: Drug Seeking and Substance Abuse
The similarities between discounting in humans and animals have led to the development of animal models of impulsive behavior based on discounting procedures. Research using such models has shown that the discounting function with delayed drug rewards (Woolverton et al., 2007) is similar to that with non-drug rewards, at least in primates (Freeman et al., 2009). In addition, the degree to which delayed food is discounted appears to be a strong predictor of drug-seeking behavior in rats and the degree of discounting has been found to increase during drug withdrawal (for a review, see Carroll et al., 2010). Thus, animal models of discounting appear to hold considerable promise for getting at the mechanisms underlying substance abuse in humans, and may provide ways to assess potential interventions directed at prevention and treatment.
It should be noted, however, that although discounting and drug-seeking may share common neural mechanisms, the logic of the relation between impulsivity and substance abuse in humans would not appear to apply to animals. That is, although human substance abusers may be discounting the long-term consequences of their behavior, it is hard to imagine how this would apply to drug-seeking in animals in most current experimental paradigms. Nevertheless, one can imagine an experimental paradigm in which animals’ drug-seeking does have delayed negative consequences, and such an approach might provide for more direct tests of current theorizing about human substance abuse.
5.6 Drug Effects on Discounting Behavior
In addition to research in which drugs serve as rewards, a number of studies have examined how drugs affect discounting of other types of rewards. For example, cocaine, nicotine, and alcohol appear to reliably result in steeper discounting of delayed food in rats (for a review, see de Wit and Mitchell, 2010). Unfortunately, for the purposes of validating animal models of the effects of drugs and other substances on discounting, most comparisons of the effects of drugs in animals and humans are confounded by differences in the type of delayed rewards (e.g., real food vs. monetary rewards). One possible approach to this problem would be to examine the effects of drugs and other substances on discounting in humans using procedures with real, directly consumable rewards (Jimura et al., 2009) that more closely approximate the procedures and rewards used with animals. Animal models of discounting have good face validity as a means to assess effects on impatience and impulsivity: with careful attention to methodological details, they offer the promise of more precise measurement of these theoretically important constructs than is possible when impatience and impulsivity are measured simply as the proportion of choices of smaller, sooner versus larger, later rewards.
6. Perinatal PCB Exposure and Deficits in Executive Function: Parallels between Animals and Humans
Appropriately designed studies in animal models provide important data that can increase our confidence in the findings from human epidemiological research. Studies assessing the impact of early developmental exposure to polychlorinated biphenyls (PCBs) on later cognitive function in the Long Evans rat provide a useful illustration of this concept. PCBs are industrial chemicals that were once widely used as dielectric fluids in capacitors and transformers. These extremely stable and lipophilic compounds became widely dispersed in the environment and are a particular problem in aquatic ecosystems where they have bioaccumulated in predatory fish at the top of the food chain. Humans who regularly consume fish from contaminated waters, such as the Great Lakes, have elevated body burdens of PCBs relative to non-fish eaters (e.g. Schantz et al., 2010).
Several epidemiological studies have followed the cognitive development of children exposed prenatally to elevated levels of PCBs (reviewed in Boucher et al., 2009). This brief review highlights the parallels between the cognitive deficits associated with prenatal PCB exposure in children reported in those studies and the cognitive deficits observed in rats exposed to PCBs perinatally. For the sake of brevity, the results from tests of two aspects of executive function—cognitive flexibility and response inhibition—are presented as examples. For a more comprehensive comparison of the cognitive domains impacted by early PCB exposure in animal models and humans see the more extensive recent review (Eubig et al., 2010).
6.1 Response inhibition
Response inhibition is the ability to either inhibit or interrupt a response during dynamic moment-to-moment behavior (Winstanley et al., 2006). In laboratory animals, response inhibition is often assessed using an operant behavior schedule known as the differential reinforcement of low rates of responding or DRL. On DRL tasks the organism must wait a specified interval of time between responses in order to receive a reinforcer. A response (e.g., a lever press) before the required interval has elapsed will reset the interval clock and delay reinforcer delivery. Recently, studies were conducted in which rats were exposed perinatally to a PCB mixture that models the PCB congener profile present in fish consumed by sports anglers in northeastern Wisconsin. Perinatal exposure to this PCB mixture resulted in decreased inter-response times and a lower ratio of reinforced: non-reinforced lever presses in a DRL schedule (Sable et al. 2009). Rice (1998) reported a similar pattern of responding in monkeys exposed during early postnatal development to a PCB mixture that included the major PCB congeners present in human breast milk. This pattern of responding is thought to be indicative of impaired response inhibition.
Interestingly, researchers studying a Great Lakes PCB cohort in Oswego, New York used a DRL task very similar to those used in the rodent and monkey studies to assess response inhibition in PCB-exposed children (Stewart et al., 2006). The children were required to wait at least 20 seconds between lever presses in order to earn marbles which they could exchange for nickels at the end of the testing period. Similar to the findings in rats (Sable et al., 2009) and monkeys (Rice, 1998), children with higher prenatal PCB exposure tended to respond too soon and, thus, earned fewer reinforcers. The consistency between the DRL results in PCB-exposed rodents, monkeys and humans is striking and increases confidence that deficits in inhibitory control are a real consequence of early PCB exposure.
In children, the ability to inhibit responding when appropriate is more often assessed by measuring commission errors (or false alarms) on continuous performance tasks (CPTs). Typical CPTs measure the child’s ability to respond (press a button) to a rare target appearing on a computer screen (e.g., the letter “X” when preceded by the letter “A” but not when preceded by other letters). Testing occurs over an extended period of time, usually 15 min or longer, and commission errors occur when the child responds to non-target stimuli. Both Great Lakes studies have reported associations between prenatal PCB exposure and deficits in response inhibition as assessed using CPTs.
In the Oswego, New York cohort, children with higher umbilical cord serum PCB levels made more errors of commission on CPTs at 4.5 (Stewart et al. 2003), 8 (Stewart et al. 2005) and 9.5 years of age (Stewart et al. 2005). The clearest evidence of a deficit was observed when the percentage of target stimuli was large relative to non-target stimuli, indicating that when the children were required to respond more frequently to targets, they were less able to inhibit responding for non-targets. In the Michigan cohort, Jacobson et al. (1992) did not observe an increase in errors of commission using a similar CPT at 4 years, but did see an increase in commission errors on a CPT at age 11 (Jacobson and Jacobson, 2003). These additional findings using a different measure of response inhibition further increase confidence that deficits in inhibitory control are associated with early PCB exposure.
6.2 Cognitive Flexibility
Cognitive flexibility is the ability to switch attention from one aspect of an object to another, or to adapt one’s responses based on situational demands, such as a change in task rules, schedule of reinforcement, or type of reinforcement (Monsell 2003). In rodents and monkeys cognitive flexibility is often assessed using discrimination-reversal learning tasks. In such tasks, the subject must choose between two possible actions in order to obtain a reinforcer. For example, in one type of spatial discrimination-reversal learning task used in rat studies, the subject must choose between two response positions (e.g., the right or left lever). Once the association is well-learned (e.g., left lever is correct) a reversal is presented in which the previously incorrect position is now associated with reinforcement (right lever is correct). The reversal evaluates how flexible the rat is in adjusting its goal-directed behavior as a result of changes in the action-outcome association. Male PCB-exposed rats learned the initial spatial discrimination at the same rate as controls, but were slower to learn the first reversal (Widholm et al. 2001). Error pattern analyses revealed that this first reversal deficit was related to the fact that the PCB-exposed rats were perseverating—that is, they were continuing to press the previously correct lever even though it was no longer associated with reinforcement (Widholm et al. 2001). Reversal learning deficits have also been reported in monkeys following developmental PCB exposure (Bowman et al. 1978; Rice 1998; Rice and Hayward 1997; Schantz et al. 1989).
In humans cognitive flexibility is often assessed using the Wisconsin Card Sorting Test (WCST), which has parallels to the discrimination-reversal learning tasks used in animals. On the WCST, subjects are asked to sort a series of cards with figures on them that differ in color, shape and number (e.g. two red squares, 3 green triangles). Each time a card is sorted, the subject receives feedback as to whether the choice was correct or incorrect and, based on this feedback, the subject must infer the correct sorting category/stimulus dimension (color, shape, or number) (Romine et al. 2004). After the subject correctly sorts the cards in a series of consecutive trials, the sorting category is changed without warning (similar to a reversal) and the subject must shift to a new sorting category. As in reversal learning, an indicator of impaired cognitive flexibility is the tendency to make perseverative errors. That is, to persist in sorting the cards by the previously correct category, even after being told repeatedly that the card placements are incorrect. The WCST was administered to children in the Michigan PCB cohort (Jacobson and Jacobson, 2003). Consistent with the findings from reversal learning tests in laboratory rats (Widholm et al., 2001), children with greater prenatal PCB exposure exhibited increased perseverative errors on the WCST, indicating that they had more difficulty changing response strategies.
7. Conclusion
In summary, these examples illustrate that findings from studies in animal models can add to the weight of evidence for associations between chemical exposures and specific cognitive outcomes in epidemiological studies. The more directly laboratory animal studies model actual human exposure scenarios, the more useful they will be in this regard. Similarly, the more closely the cognitive tests used in animal studies parallel those used in humans in terms of the functional domains assessed, the more useful those studies will be.
Highlights.
Identical cognitive assessment tools for laboratory animals and humans.
Memory development in monkeys useful in identifying relevant brain areas.
Cognitive dysfunction of Parkinsonism is modeled in a monkey model.
Delay discounting procedures are reasonable surrogates of ‘impulsivity.’
PCB-induced cognitive deficits in rats parallel those seen in children.
Acknowledgements
Grant sponsor: NIMH; Grant Number: MH-58846; Grant Sponsor: NICHD; Grant Number: HD-35471; Grant Sponsor NIH; Grant Number MH-055308, RR-00165. The findings and conclusions in the report by M.G. Paule are those of the author and do not necessarily represent the views of the FDA and mention of trade names or commercial products does not constitute endorsement or recommendation for use.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarado MC, Bachevalier J. Comparison of the Effects of Damage to the Perirhinal and Parahippocampal Cortex on Transverse Patterning and Location Memory in Rhesus Macaques. J Neurosci. 2005;25:1599–1609. doi: 10.1523/JNEUROSCI.4457-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado MC, Bachevalier J. Revisiting the maturation of medial temporal lobe memory functions in primates. Learn Mem. 2000;7:244–256. doi: 10.1101/lm.35100. [DOI] [PubMed] [Google Scholar]
- Alvarado MC, Rudy JW. A comparison of kainic acid plus colchicine and ibotenic acid-induced hippocampal formation damage on four configural tasks in rats. Behav Neurosci. 1995 doi: 10.1037//0735-7044.109.6.1052. 1091052-62. [DOI] [PubMed] [Google Scholar]
- Alvarado MC, Rudy JW. Some properties of configural learning: an investigation of the transverse-patterning problem. J Exp Psychol Anim Behav Process. 1992;18:145–153. doi: 10.1037//0097-7403.18.2.145. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Nemanic S. Memory for spatial location and object-place associations are differently processed by the hippocampal formation, parahippocampal areas TH/TF and perirhinal cortex. Hippocampus. 2008;18:64–80. doi: 10.1002/hipo.20369. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Vargha-Khadem F. The primate hippocampus: ontogeny, early insult and memory. Curr Opin Neurobiol. 2005;15:168–174. doi: 10.1016/j.conb.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Marsch LA. Toward a behavioral economic understanding of drug dependence: Delay discounting processes. Addiction. 2001;96:73–86. doi: 10.1046/j.1360-0443.2001.961736.x. [DOI] [PubMed] [Google Scholar]
- Blue S, Kazama A, Bachevalier J. The normal development of object-place association memory is altered by neonatal hippocampal lesions in rhesus monkeys. Neuroscience Meeting Planner; Society for Neuroscience Program; Chicago, IL. 2009. 7 Online. [Google Scholar]
- Boucher O, Muckle G, Bastien CH. Prenatal exposure to polychlorinated biphenyls: A neuropsychologic analysis. Environ Hlth Perspect. 2009;117(1):7–16. doi: 10.1289/ehp.11294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman RE, Heironimus MP, Allen JR. Correlation of PCB body burden with behavioral toxicology in monkeys. Pharmacol Biochem Behav. 1978;9(1):49–56. doi: 10.1016/0091-3057(78)90012-6. [DOI] [PubMed] [Google Scholar]
- Brown RG, Marsden CD. Cognitive function in Parkinson’s disease: from description to theory. Trends Neurosci. 1990;13:21–29. doi: 10.1016/0166-2236(90)90058-i. [DOI] [PubMed] [Google Scholar]
- Brun VH, Otnass MK, Molden S, Steffenach HA, Witter MP, Moser MB, Moser EI. Place cells and place recognition maintained by direct entorhinal-hippocampal circuitry. Science. 2002;296:2243–2246. doi: 10.1126/science.1071089. [DOI] [PubMed] [Google Scholar]
- Buffalo EA, Ramus SJ, Clark RE, Teng E, Squire LR, Zola SM. Dissociation between the effects of damage to perirhinal cortex and area TE. Learn Mem. 1999;6:572–599. doi: 10.1101/lm.6.6.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert AL, Green L, Myerson J. Delay discounting of qualitatively different reinforcers in rats. J Exp Anal Behav. 2010;93:171–184. doi: 10.1901/jeab.2010.93-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Anker JJ, Mach JL, Newman JL, Perry JL. Delay discounting as a predictor of drug abuse. In: Madden GJ, Bickel WK, editors. Impulsivity: The behavioral and neurological science of discounting. Washington, DC: American Psychological Association; 2010. pp. 243–271. [Google Scholar]
- Chelonis JJ, Daniels-Shaw JL, Blake DJ, Paule MG. Developmental aspects of delayed matching-to-sample task performance in children. Neurotox Teratol. 2000;22(5):683–694. doi: 10.1016/s0892-0362(00)00090-8. [DOI] [PubMed] [Google Scholar]
- Chelonis JJ, Edwards MC, Schulz EG, Baldwin R, Blake DJ, Wenger A, Paule MG. Stimulant medication improves recognition memory in children diagnosed with attention deficit/hyperactivity disorder. Exp Clin Psychopharm. 2002;10:400–407. doi: 10.1037//1064-1297.10.4.400. [DOI] [PubMed] [Google Scholar]
- Chelonis JJ, Gillam MP, Paule MG. The effects of prenatal cocaine exposure on reversal learning using a simple visual discrimination task in rhesus monkeys. Neurotox Teratol. 2003;25:437–446. doi: 10.1016/s0892-0362(03)00017-5. [DOI] [PubMed] [Google Scholar]
- Chelonis JJ, Flake RA, Baldwin RL, Blake DJ, Paule MG. Developmental aspects of timing behavior in children. Neurotox Teratol. 2004;26(3):461–476. doi: 10.1016/j.ntt.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Chelonis JJ, Gravelin CR, Paule MG. Assessing Motivation in Children Using a Progressive Ratio Task. Behav Proc. 2011;87:203–209. doi: 10.1016/j.beproc.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Cools R, Barker RA, Sahakian BJ, Robbins TW. Enhanced or impaired cognitive function in Parkinson’s disease as a function of dopaminergic medication and task demands. Cereb Cortex. 2001;11:1136–1143. doi: 10.1093/cercor/11.12.1136. [DOI] [PubMed] [Google Scholar]
- Cooper JA, Sagar HJ, Jordan N, Harvey NS, Sullivan EV. Cognitive impairment in early, untreated Parkinson's disease and its relationship to motor disability. Brain. 1991;114:2095–2122. doi: 10.1093/brain/114.5.2095. [DOI] [PubMed] [Google Scholar]
- Daniels-Shaw JL, Blake DJ, Paule MG, Chelonis JJ. The effects of reinforcer type on performance of a delayed matching-to-sample task. Proceed Assoc Behav Anal. 2001 [Google Scholar]
- Decamp E, Schneider JS. Attention and executive function deficits in chronic low dose MPTP-treated non-human primates. Eur J Neurosci. 2004;20:1371–1378. doi: 10.1111/j.1460-9568.2004.03586.x. [DOI] [PubMed] [Google Scholar]
- Decamp E, Tinker JP, Schneider JS. Attentional cueing reverses deficits in spatial working memory task performance in chronic low dose MPTP-treated monkey. Behav Brain Res. 2001;152:259–262. doi: 10.1016/j.bbr.2003.10.007. [DOI] [PubMed] [Google Scholar]
- de Wit H, Mitchell SH. Drug effects on delay discounting. In: Madden GJ, Bickel WK, editors. Impulsivity: The behavioral and neurological science of discounting. Washington, DC: American Psychological Association; 2010. pp. 213–241. [Google Scholar]
- Downes JJ, Roberts AC, Sahakian BJ, Evenden JL, Morris RG, Robbins TW. Impaired extra-dimensional shift performance in medicated and unmedicated Parkinson's disease: evidence for a specific attentional dysfunction. Neuropsychologia. 1989;27:1329–1343. doi: 10.1016/0028-3932(89)90128-0. [DOI] [PubMed] [Google Scholar]
- Estle SJ, Green L, Myerson J, Holt DD. Discounting of monetary and directly consumable rewards. Psychol Sci. 2007;18:58–63. doi: 10.1111/j.1467-9280.2007.01849.x. [DOI] [PubMed] [Google Scholar]
- Eubig PA, Aguiar A, Schantz SL. Lead and PCBs as risk factors for attention deficit hyperactivity disorder. Environ Hlth Persp. 2010;118:1654–1667. doi: 10.1289/ehp.0901852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan JF. Memory in the infant. J Exp Child Psychol. 1970;9:217–226. doi: 10.1016/0022-0965(70)90087-1. [DOI] [PubMed] [Google Scholar]
- Fernandez HH, Crucian GP, Okun MS, Price CC, Bowers D. Mild cognitive impairment in Parkinson’s disease: the challenge and the promise. Neuropsychiatr Dis Treat. 2005;1:37–50. doi: 10.2147/nedt.1.1.37.52295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flowers KA, Robertson C. The effect of Parkinson's disease on the ability to maintain a mental set. J Neurol Neurosurg Psychiatry. 1985;48:517–529. doi: 10.1136/jnnp.48.6.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman M, Oscar-Berman M. Selective delayed response deficits in Parkinson's and Alzheimer's disease. Arch Neurol. 1986;43:886–890. doi: 10.1001/archneur.1986.00520090026011. [DOI] [PubMed] [Google Scholar]
- Freeman KB, Green L, Myerson J, Woolverton WL. Delay discounting of saccharin in rhesus monkeys. Behav Proc. 2009;82:214–218. doi: 10.1016/j.beproc.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L, Myerson J. Experimental and correlational analyses of delay and probability discounting. In: Madden GJ, Bickel WK, editors. Impulsivity: The behavioral and neurological science of discounting. Washington, DC: American Psychological Association; 2010. pp. 67–92. [Google Scholar]
- Green L, Myerson J, Shah AK, Estle SJ, Holt DD. Do adjusting-amount and adjusting-delay procedures produce equivalent estimates of subjective value in pigeons? J Exp Anal Behav. 2007;87:337–347. doi: 10.1901/jeab.2007.37-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L, Myerson J, Holt DD, Slevin JR, Estle SJ. Discounting of delayed food rewards in pigeons and rats: Is there a magnitude effect? J Exp Anal Beha. 2004;81:39–50. doi: 10.1901/jeab.2004.81-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L, Myerson J. A discounting framework for choice with delayed and probabilistic rewards. Psychol Bull. 2004;130:769–792. doi: 10.1037/0033-2909.130.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goursaud AP, Mendoza SP, Capitanio JP. Do neonatal bilateral ibotenic acid lesions of the hippocampal formation or of the amygdala impair HPA axis responsiveness and regulation in infant rhesus macaques (Macaca mulatta)? Brain Res. 2006;1071:97–104. doi: 10.1016/j.brainres.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Gunderson VM, Grant KS, Burbacher TM, Fagan JF, Mottet NK. The effect of low-level prenatal methylmercury exposure on visual recognition memory in infant crab-eating macaques. Child Dev. 1988;57:1076–1083. [PubMed] [Google Scholar]
- Hashemi RR, Pearce BA, Hinson WG, Paule MG, Young JF. IQ estimation of monkeys based on human data using rough sets. Proceed 3rd Intl Wrkshp Rough Sets Soft Comp. 1994:400–407. [Google Scholar]
- Hietanen M, Teravainen H. Cognitive performance in early Parkinson's disease. Acta Neurol Scand. 1986;73:151–159. doi: 10.1111/j.1600-0404.1986.tb03257.x. [DOI] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW. Prenatal exposure to polychlorinated biphenyls and attention at school age. J Pediatr. 2003;143(6):780–788. doi: 10.1067/S0022-3476(03)00577-8. [DOI] [PubMed] [Google Scholar]
- Jimura K, Myerson J, Hilgard J, Braver TS, Green L. Are people really more patient than other animals? Evidence from human discounting of real liquid rewards. Psychon Bull Rev. 2009;16:1071–1075. doi: 10.3758/PBR.16.6.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulisevsky J. Role of dopamine in learning and memory: Implications for the treatment of cognitive dysfunction in patients with Parkinson’s disease. Drugs Aging. 2000;16:365–379. doi: 10.2165/00002512-200016050-00006. [DOI] [PubMed] [Google Scholar]
- Kumaran D, Hassabis D, Spiers HJ, Vann SD, Vargha-Khadem F, Maguire EA. Impaired spatial and non-spatial configural learning in patients with hippocampal pathology. Neuropsychologia. 2007;45:2699–2711. doi: 10.1016/j.neuropsychologia.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange KW, Robbins TW, Marsden CD, James M, Owen AM, Paul GM. L-Dopa withdrawal in Parkinson's disease selectively impairs cognitive performance in tests sensitive to frontal lobe dysfunction. Psychopharmacol. 1992;107:394–404. doi: 10.1007/BF02245167. [DOI] [PubMed] [Google Scholar]
- Lavenex P, Banta Lavenex P, Amaral DG. Postnatal development of the primate hippocampal formation. Dev Neurosci. 2007;29:179–192. doi: 10.1159/000096222. [DOI] [PubMed] [Google Scholar]
- Levin BE, Llabre MM, Weiner WJ. Cognitive impairments associated with early Parkinson's disease. Neurology. 1989;39:557–561. doi: 10.1212/wnl.39.4.557. [DOI] [PubMed] [Google Scholar]
- Lewis SJG, Dove A, Robbins TW, Barker RA, Owen AM. Striatal contributions to working memory: a functional magnetic resonance imaging study in humans. Eur J Neurosci. 2004;19:755–760. doi: 10.1111/j.1460-9568.2004.03108.x. [DOI] [PubMed] [Google Scholar]
- Lewis SJG, Slabosz A, Robbins TW, Barker RA, Owen AM. Dopaminergic basis for deficits in working memory but not attentional set-shifting in Parkinson's disease. Neuropsychologia. 2005;43:823–832. doi: 10.1016/j.neuropsychologia.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Madden GJ, Johnson PS. A delay-discounting primer. In: Madden GJ, Bickel WK, editors. Impulsivity: The behavioral and neurological science of discounting. Washington, DC: American Psychological Association; 2010. pp. 11–37. [Google Scholar]
- Malkova L, Bachevalier J, Mishkin M, Saunders RC. Neurotoxic lesions of perirhinal cortex impair visual recognition memory in rhesus monkeys. Neuroreport. 2001;12:1913–1917. doi: 10.1097/00001756-200107030-00029. [DOI] [PubMed] [Google Scholar]
- Mamikonyan E, Moberg PJ, Siderowf A, Duda JE, Ten Have T, Hurtig HI, Stern MB, Weintraub D. Mild cognitive impairment is common in Parkinson’s disease patients with normal mini-mental state exam (MMSE) scores. Parkinsonism Rel Dis. 2009;15:226–231. doi: 10.1016/j.parkreldis.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur JE. An adjusting procedure for studying delayed reinforcement. In: Commons ML, Mazur JE, Nevin JA, Rachlin H, editors. Quantitative analyses of behavior: Vol: 5. The effects of delay and of intervening events on reinforcement value. Hillsdale, NJ: Erlbaum; 1987. pp. 55–73. [Google Scholar]
- Mazur JE. Tradeoffs among delay, rate, and amount of reinforcement. Behav Proc. 2000;49:1–10. doi: 10.1016/s0376-6357(00)00070-x. [DOI] [PubMed] [Google Scholar]
- McKee RD, Squire LR. On the development of declarative memory. J Exp Psychol Learn Mem Cogn. 1993;19:397–404. doi: 10.1037//0278-7393.19.2.397. [DOI] [PubMed] [Google Scholar]
- Monsell S. Task switching. Trends Cogn Sci. 2003;7(3):134–140. doi: 10.1016/s1364-6613(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Morris P, Binienda Z, Gillam MP, Klein J, McMartin K, Koren G, Duhart HM, Slikker W, Jr, Paule MG. The effect of chronic cocaine exposure throughout pregnancy on maternal and infant outcomes in the rhesus monkey. Neurotox Teratol. 1997;19(1):47–57. doi: 10.1016/s0892-0362(96)00187-0. [DOI] [PubMed] [Google Scholar]
- Muñoz M, Chadwick M, Perez-Hernandez E, Vargha-Khadem R, Mishkin M. Novelty preference in patients with developmental amnesia. Hippocampus. 2010;21:1268–1276. doi: 10.1002/hipo.20836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myerson J, Green L, Hanson JS, Holt DD, Estle SJ. Discounting delayed and probabilistic rewards: Processes and traits. J Econ Psychol. 2003;24:619–635. [Google Scholar]
- Nemanic S, Alvarado MC, Bachevalier J. The hippocampal/parahippocampal regions and recognition memory: insights from visual paired comparison versus object-delayed nonmatching in monkeys. J Neurosci. 2004;24:2013–2026. doi: 10.1523/JNEUROSCI.3763-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieoullon A. Dopamine and the regulation of cognition and attention. Prog Neurobiol. 2002;67:53–83. doi: 10.1016/s0301-0082(02)00011-4. [DOI] [PubMed] [Google Scholar]
- Nieoullon A, Coquerel A. Dopamine: a key regulator to adapt action, emotion, motivation and cognition. Curr Opin Neurol. 2003;16 Suppl 2:S3–S9. [PubMed] [Google Scholar]
- Odum AL, Rainaud CP. Discounting of delayed hypothetical money, alcohol, and food. Behav Proc. 2003;64:305–313. doi: 10.1016/s0376-6357(03)00145-1. [DOI] [PubMed] [Google Scholar]
- Oken E, Wright RO, Kleinman KP, Bellinger D, Amarasiriwardena CJ, Hu H, Rich-Edwards JW, Gillman MW. Maternal fish consumption, hair mercury, and infant cognition in a U.S. cohort. Environ Hlth Persp. 2005;113:1376–1380. doi: 10.1289/ehp.8041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olanow CW, Stern MB, Sethi K. The scientific and clinical basis for the treatment of Parkinson disease. Neurology. 2009;72 Suppl 4:S70–S79. doi: 10.1212/WNL.0b013e3181a1d44c. [DOI] [PubMed] [Google Scholar]
- Overman WH, Bachevalier J, Schuhmann E, Ryan P. Cognitive gender differences in very young children parallel biologically based cognitive gender differences in monkeys. Behav Neurosci. 1996;110:673–684. doi: 10.1037//0735-7044.110.4.673. [DOI] [PubMed] [Google Scholar]
- Owen AM, Beksinska M, James M, Leigh PN, Summers BA, Marsden CD, Quinn NP, Sahakian BJ, Robbins TW. Visuospatial memory deficits at different stages of Parkinson's disease. Neuropsychologia. 1993;7:627–644. doi: 10.1016/0028-3932(93)90135-m. [DOI] [PubMed] [Google Scholar]
- Owen AM, James M, Leigh PN, Summers BA, Mardsen CD, Quinn NP, Lange KW, Robbins TW. Fronto-striatal cognitive deficits at different stages of Parkinson's disease. Brain. 1992;115:1727–1751. doi: 10.1093/brain/115.6.1727. [DOI] [PubMed] [Google Scholar]
- Owen AM, Iddon JL, Hodges JR, Summers BA, Robbins TW. Spatial and non-spatial working memory at different stages of Parkinson's disease. Neuropsychologia. 1997;35:519–532. doi: 10.1016/s0028-3932(96)00101-7. [DOI] [PubMed] [Google Scholar]
- Paule MG. Use of the NCTR operant test battery in nonhuman primates. Neurotox Teratol. 1990;12(5):413–418. doi: 10.1016/0892-0362(90)90002-t. [DOI] [PubMed] [Google Scholar]
- Paule MG, Forrester TM, Maher MA, Cranmer JM, Allen RR. Monkey versus human performance in the NCTR operant test battery. Neurotox Teratol. 1990;12(5):503–507. doi: 10.1016/0892-0362(90)90014-4. [DOI] [PubMed] [Google Scholar]
- Paule MG, Allen RR, Bailey JR, Scallet AC, Ali SF, Brown RM, Slikker W., Jr Chronic marijuana smoke exposure in the rhesus monkey II: Effects on progressive ratio and conditioned position responding. J Pharmacol Exp Therap. 1992;260(1):210–222. [PubMed] [Google Scholar]
- Paule MG, Chelonis JJ, Buffalo EA, Blake DJ, Casey PH. Operant test battery performance in children: correlation with IQ. Neurotox Teratol. 1999;21(3):223–230. doi: 10.1016/s0892-0362(98)00045-2. [DOI] [PubMed] [Google Scholar]
- Paule MG, Li M, Allen RR, Liu F, Zou X, Hotchkiss C, Hanig JP, Patterson TA, Slikker W, Jr, Wang C. Ketamine anesthesia during the first week of life can cause long-lasting cognitive deficits in rhesus monkeys. Neurotox Teratol. 2011;33:220–230. doi: 10.1016/j.ntt.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne C, Machado CJ, Bliwise NG, Bachevalier J. Maturation of the hippocampal formation and amygdala in Macaca mulatta: a volumetric magnetic resonance imaging study. Hippocampus. 2010;20:922–935. doi: 10.1002/hipo.20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popke EJ, Allen RR, Pearson EC, Hammond TG, Paule MG. Differential effects of two NMDA receptor antagonists on cognitive-behavioral development in non human primates I. Neurotox Teratol. 2001a;23:319–332. doi: 10.1016/s0892-0362(01)00156-8. [DOI] [PubMed] [Google Scholar]
- Popke EJ, Allen RR, Pearson EC, Hammond TG, Paule MG. Differential effects of two NMDA receptor antagonists on cognitive-behavioral performance in young non-human primates II. Neurotox Teratol. 2001b;23:333–347. doi: 10.1016/s0892-0362(01)00138-6. [DOI] [PubMed] [Google Scholar]
- Postle BR, Jonides J, Smith E, Corkin S, Growdon JH. Spatial, but not object, delayed response is impaired in early Parkinson's disease. Neuropsychology. 1997;11:171–179. doi: 10.1037//0894-4105.11.2.171. [DOI] [PubMed] [Google Scholar]
- Reynolds B. A review of delay-discounting research with humans: Relations to drug use and gambling. Behav Pharmacol. 2006;17:651–667. doi: 10.1097/FBP.0b013e3280115f99. [DOI] [PubMed] [Google Scholar]
- Rice DC. Effects of postnatal exposure of monkeys to a PCB mixture on spatial discrimination reversal and DRL performance. Neurotox Teratol. 1998;20(4):391–400. doi: 10.1016/s0892-0362(97)00134-7. [DOI] [PubMed] [Google Scholar]
- Rice DC, Hayward S. Effects of postnatal exposure to a PCB mixture in monkeys on nonspatial discrimination reversal and delayed alternation performance. Neurotoxicology. 1997;18(2):479–494. [PubMed] [Google Scholar]
- Richards JB, Mitchell SH, de Wit H, Seiden LS. Determination of discount functions in rats with an adjusting-amount procedure. J Exp Anal Behav. 1997;67:353–366. doi: 10.1901/jeab.1997.67-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond J, Nelson CA. Accounting for change in declarative memory: a cognitive neuroscience perspective. Devel Rev. 2007;27:349–373. doi: 10.1016/j.dr.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickard TC, Verfaellie M, Grafman J. Transverse Patterning and Human Amnesia. J Cogn Neurosci. 2006;18:1723–1733. doi: 10.1162/jocn.2006.18.10.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW, Keith JR, Georgen K. The effect of age on children’s learning of problems that require a configural association solution. Devel Psychobiol. 1993;26:171–184. doi: 10.1002/dev.420260304. [DOI] [PubMed] [Google Scholar]
- Sable HJ, Eubig PA, Powers BE, Wang VC, Schantz SL. Developmental exposure to PCBs and/or MeHg: Effects on a differential reinforcement of low rates (DRL) operant task before and after amphetamine drug challenge. Neurotox Teratol. 2009;31(3):149–158. doi: 10.1016/j.ntt.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawamoto N, Honda M, Hanakawa T, Fukuyama H, Shibasaki H. Cognitive slowing in Parkinson’s disease: a behavioral evaluation independent of motor slowing. J Neurosci. 2002;22:5198–5203. doi: 10.1523/JNEUROSCI.22-12-05198.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schantz SL, Levin ED, Bowman RE, Heironimus MP, Laughlin NK. Effects of perinatal PCB exposure on discrimination-reversal learning in monkeys. Neurotox Teratol. 1989;11(3):243–250. doi: 10.1016/0892-0362(89)90066-4. [DOI] [PubMed] [Google Scholar]
- Schantz SL, Gardiner JC, Aguiar A, Tang X, Gasior DM, Sweeney AM, Peck JD, Gillard D, Kostyniak PJ. Contaminant profiles in Southeast Asian immigrants consuming fish from polluted waters in northeastern Wisconsin. Environ Res. 2010;110(1):33–39. doi: 10.1016/j.envres.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JS, Roeltgen DP. Delayed matching-to-sample, object retrieval, and discrimination reversal deficits in chronic low dose MPTP-treated monkeys. Brain Res. 1993;615:351–354. doi: 10.1016/0006-8993(93)90049-s. [DOI] [PubMed] [Google Scholar]
- Schneider JS, Kovelwski CJ. Chronic exposure to low doses of MPTP. I. Cognitive deficits in motor asymptomatic monkeys. Brain Res. 1990;519:122–128. doi: 10.1016/0006-8993(90)90069-n. [DOI] [PubMed] [Google Scholar]
- Schulze GE, McMillan DE, Bailey JR, Scallet AC, Ali SF, Slikker W, Jr, Paule MG. Acute effects of delta-9-tetrahydrocannabinol (THC) in rhesus monkeys as measured by performance in a battery of cognitive function tests. J Pharmacol Exp Therap. 1988;245(1):178–186. [PubMed] [Google Scholar]
- Seress L. Comparative anatomy of the hippocampal dentate gyrus in adult and developing rodents, non-human primates and humans. Prog Brain Res. 2007;163:23–41. doi: 10.1016/S0079-6123(07)63002-7. [DOI] [PubMed] [Google Scholar]
- Seress L, Abraham H, Tornoczky T, Kosztolanyi G. Cell formation in the human hippocampal formation from mid-gestation to the late postnatal period. Neuroscience. 2001;105:831–843. doi: 10.1016/s0306-4522(01)00156-7. [DOI] [PubMed] [Google Scholar]
- Seress L, Ribak CE. Postnatal development of CA3 pyramidal neurons and their afferents in the Ammon's horn of rhesus monkeys. Hippocampus. 1995;5:217–231. doi: 10.1002/hipo.450050308. [DOI] [PubMed] [Google Scholar]
- Sharpe MH. Distractibility in early Parkinson's disease. Cortex. 1990;26:239–246. doi: 10.1016/s0010-9452(13)80353-x. [DOI] [PubMed] [Google Scholar]
- Sharpe MH. Auditory attention in early Parkinson's disease: an impairment in focused attention. Neuropsychologia. 1992;30:101–106. doi: 10.1016/0028-3932(92)90019-i. [DOI] [PubMed] [Google Scholar]
- Spiers HJ, Burgess N, Hartley T, Vargha-Khadem F, O'Keefe J. Bilateral hippocampal pathology impairs topographical and episodic memory but not visual pattern matching. Hippocampus. 2001;11:715–725. doi: 10.1002/hipo.1087. [DOI] [PubMed] [Google Scholar]
- Stern Y, Mayeux R, Cote L. Reaction time and vigilance in Parkinson's disease: Possible role of altered norepinephrine metabolism. Arch Neurol. 1984;41:1086–1089. doi: 10.1001/archneur.1984.04050210084021. [DOI] [PubMed] [Google Scholar]
- Stewart P, Fitzgerald S, Reihman J, Gump B, Lonky E, Darvill T, Pagano J, Hauser P. Prenatal PCB exposure, the corpus callosum, and response inhibition. Environ Hlth Perspect. 2003;111(13):1670–1677. doi: 10.1289/ehp.6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart P, Reihman J, Gump B, Lonky E, Darvill T, Pagano J. Response inhibition at 8 and 9 1/2 years of age in children prenatally exposed to PCBs. Neurotox Teratol. 2005;27(6):771–780. doi: 10.1016/j.ntt.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Stewart PW, Sargent DM, Reihman J, Gump BB, Lonky E, Darvill T, Hicks H, Pagano J. Response inhibition during differential reinforcement of low rates (DRL) schedules may be sensitive to low-level polychlorinated biphenyl, methylmercury, and lead exposure in children. Environ Hlth Perspect. 2006;114(12):1923–1929. doi: 10.1289/ehp.9216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sybirska E, Davachi L, Goldman-Rakic PS. Prominence of direct entorhinal-CA1 pathway activation in sensorimotor and cognitive tasks revealed by 2-DG functional mapping in nonhuman primate. J Neurosci. 2000;20:5827–5834. doi: 10.1523/JNEUROSCI.20-15-05827.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbaan D, Marinus J, Visser M, van Rooden SM, Stiggelbout AM, Middlekoop HAM, van Hilten JJ. Cognitive impairment in Parkinson’s disease. J Neurol Neurosurg Psychiat. 2007;78:1182–1187. doi: 10.1136/jnnp.2006.112367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widholm JJ, Clarkson GB, Strupp BJ, Crofton KM, Seegal RF, Schantz SL. Spatial reversal learning in Aroclor 1254-exposed rats: Sex-specific deficits in associative ability and inhibitory control. Tox Appl Pharm. 2001;174(2):188–198. doi: 10.1006/taap.2001.9199. [DOI] [PubMed] [Google Scholar]
- Williams SM, Goldman-Rakic PS. Widespread origin of the primate mesofrontal dopamine system. Cereb Cortex. 1998;8:321–345. doi: 10.1093/cercor/8.4.321. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Myerson J, Green L. Delay discounting of cocaine by rhesus monkeys. Exp Clin Psychopharm. 2007;15:238–244. doi: 10.1037/1064-1297.15.3.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalonis I, Christidi F, Zournas C, Ilias A, Kararizou E, Anagnostouli M, Andreadou E, Vassilopoulos D. Cognitive dysfunction in non-demented patients with Parkinson’s disease. Ann Gen Psychiatr. 2010;9 Suppl 1:S146–S147. [Google Scholar]
- Zeamer A, Alvarado MC, Bachevalier J. Development of medial temoporal lobe processes in non-human primates. In: Blumber MS, Freeman JH, Robinson SR, editors. Oxford Handbook of Developmental Behavioral Neuroscience. New York, New York: Oxford University Press, Inc.; 2010. pp. 607–616. [Google Scholar]
- Zeamer A, Bachevalier J. Neonatal lesions of the perirhinal cortex alter the development of object recognition memory abilities in monkeys. Neuroscience Meeting Planner; Society for Neuroscience; Chicago, IL. 2009. Online, Program No. 98.6. [Google Scholar]
- Zeamer A, Heuer E, Bachevalier J. Developmental trajectory of object recognition memory in infant rhesus macaques with and without neonatal hippocampal lesions. J Neurosci. 2010;30:9157–9165. doi: 10.1523/JNEUROSCI.0022-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola SM, Squire LR, Teng E, Stefanacci L, Buffalo EA, Clark RE. Impaired recognition memory in monkeys after damage limited to the hippocampal region. J Neurosci. 2000;20:451–463. doi: 10.1523/JNEUROSCI.20-01-00451.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]