Abstract
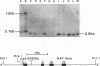
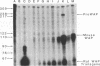
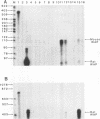
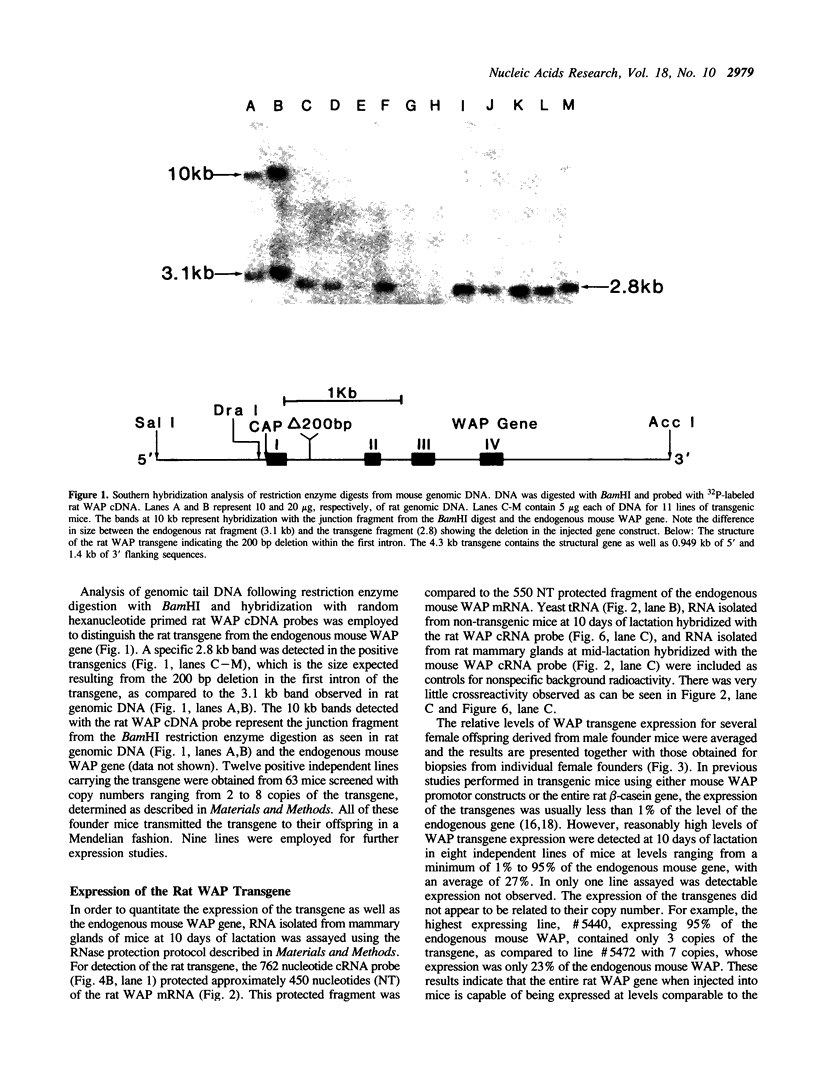
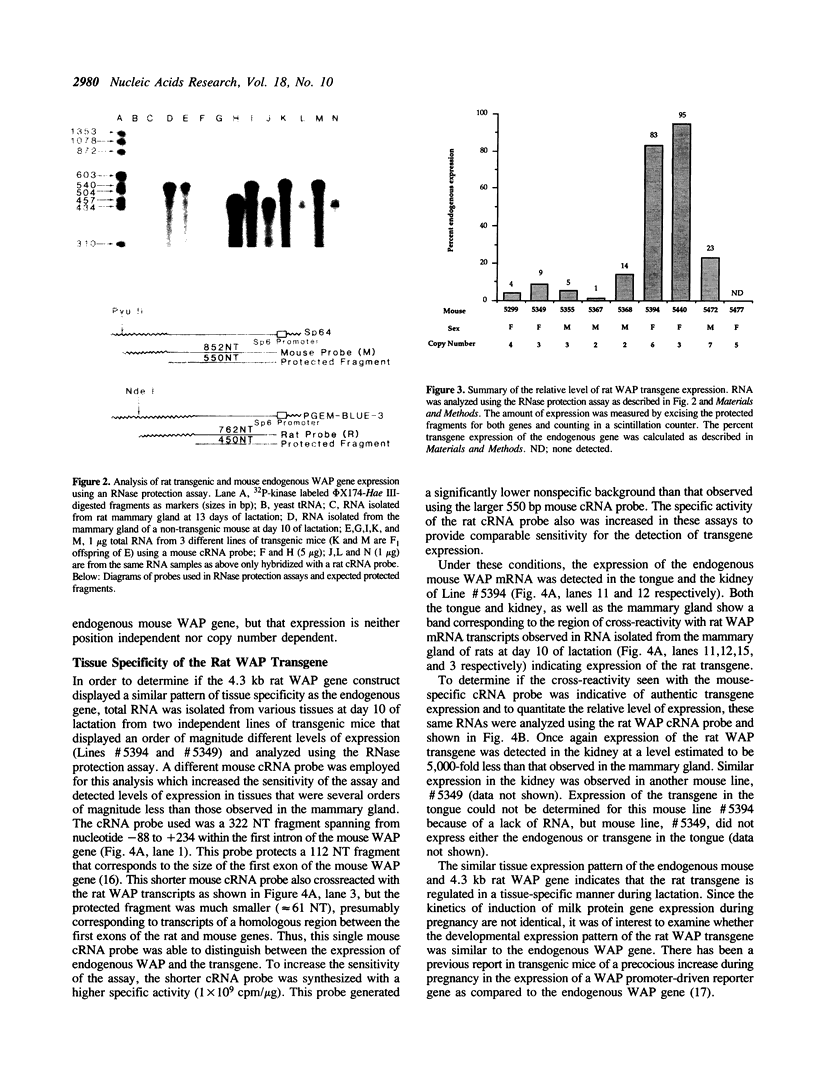
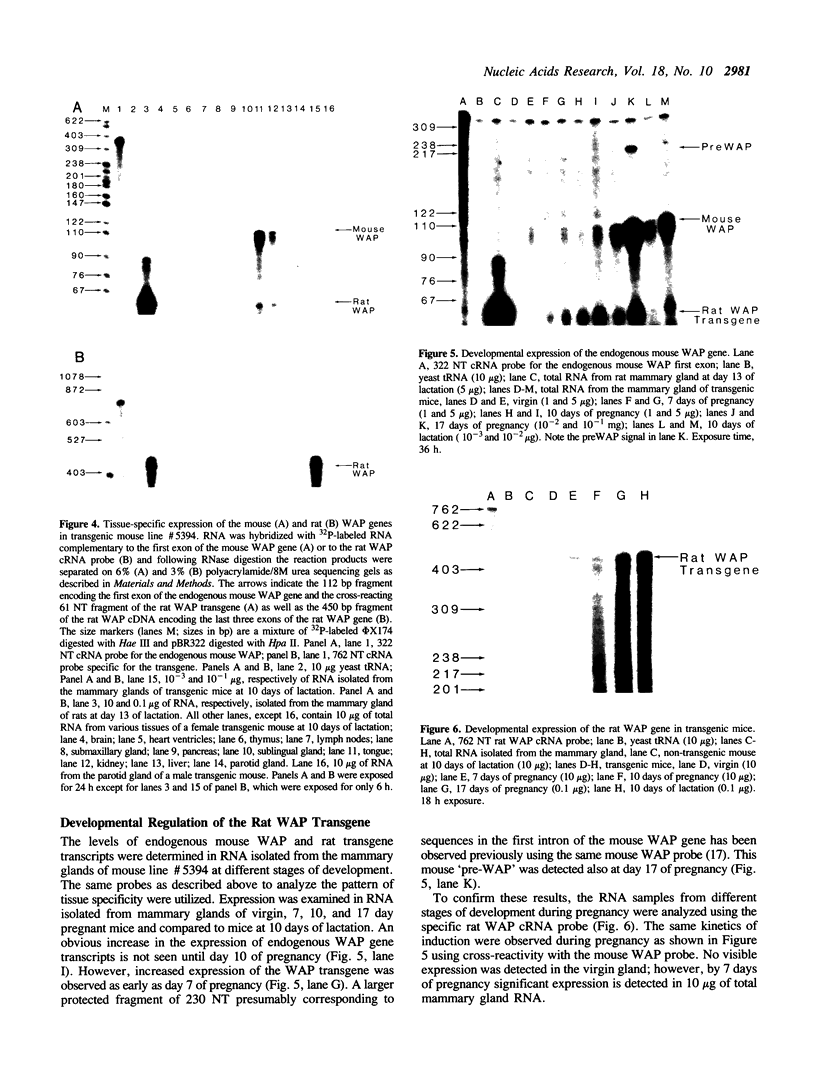
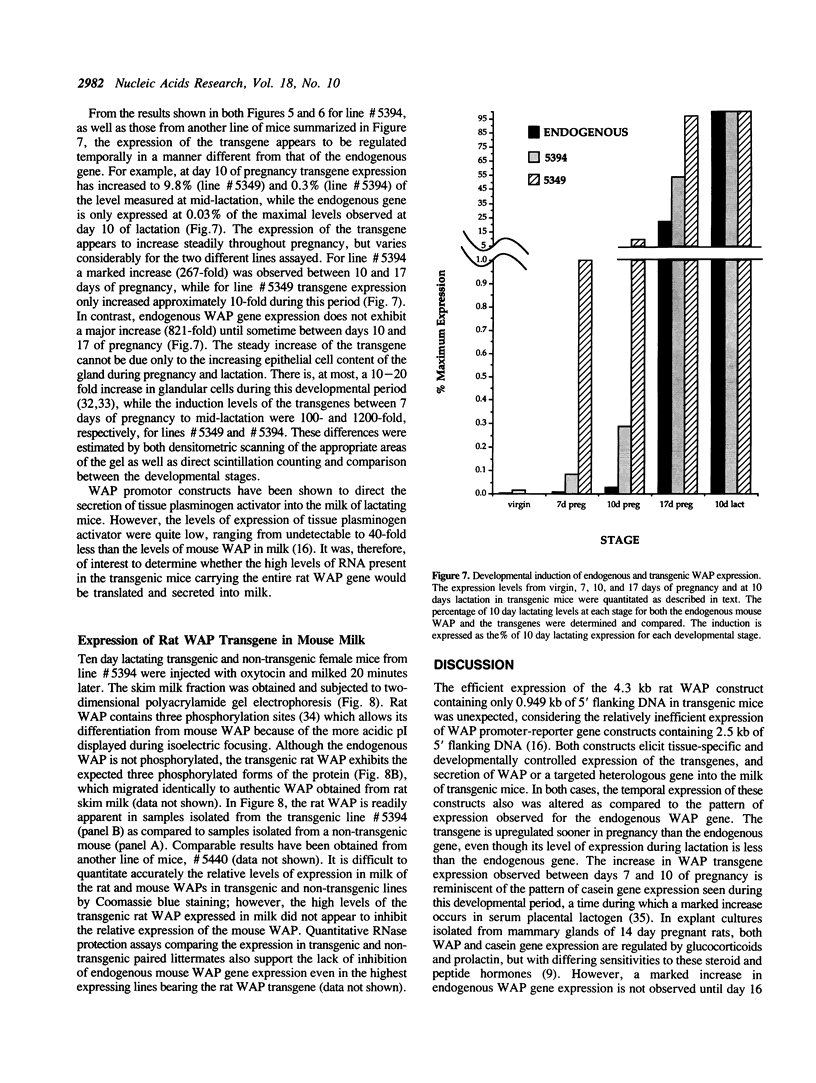
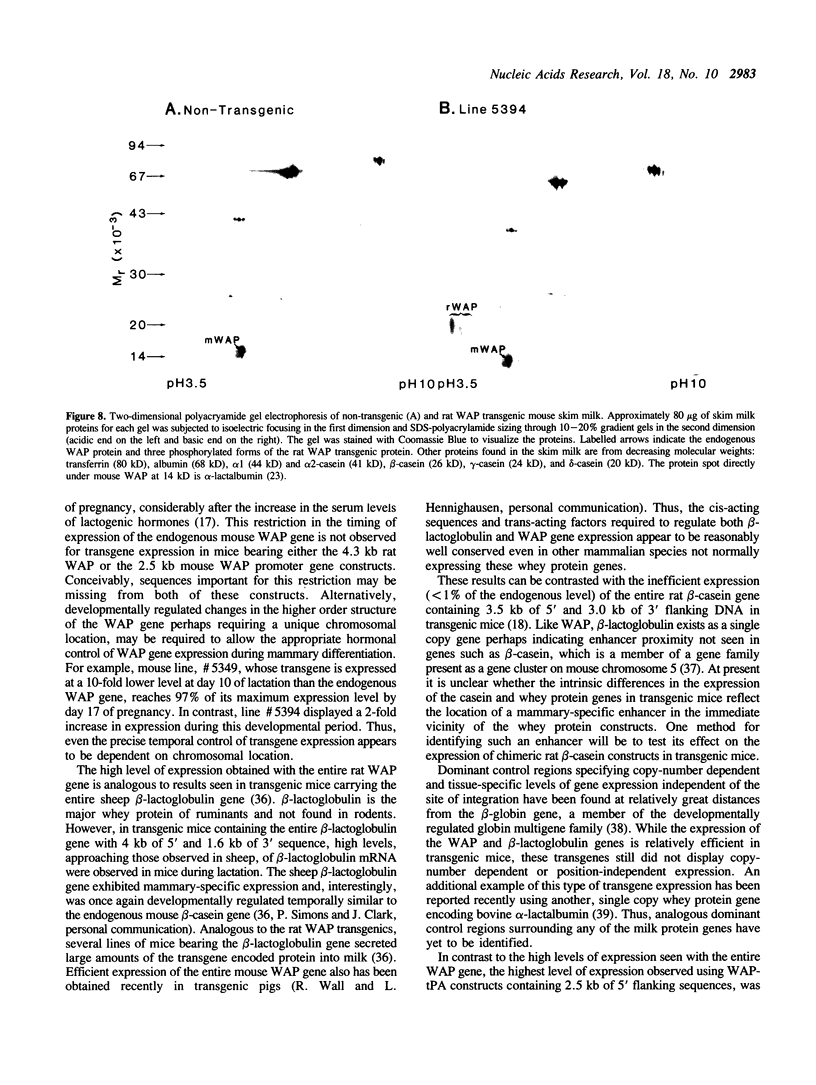
The importance of intragenic and 3' flanking sequences in the control of the temporal, hormonal and tissue-specific expression of milk whey acidic protein (WAP) has been demonstrated in transgenic mice. Mouse lines carrying a 4.3 kb genomic clone containing the entire rat WAP gene minus 200 bp of the first intron with 0.949 kb of 5' and 1.4 kb of 3' flanking DNA were generated. In eight of nine independent lines of mice analyzed, WAP transgene expression was detected at levels ranging from 1% to 95% (average, 27%) of the endogenous gene. The transgene was expressed preferentially in the mammary gland. Although developmentally regulated during pregnancy and lactation, the temporal pattern of WAP transgene expression differed from the endogenous gene. A precocious increase in expression of the transgene was detected at 7 days of pregnancy, several days earlier in pregnancy than the major increase observed in endogenous mouse WAP mRNA. The rat WAP transgene was translated and secreted into the milk of transgenic mice at levels comparable to the endogenous mouse WAP. This is the first report of a gene that is negatively regulated in dissociated cell cultures as well as in transfected cells, yet is expressed efficiently in the correct multicellular environment of the transgenic mouse.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andres A. C., Schönenberger C. A., Groner B., Hennighausen L., LeMeur M., Gerlinger P. Ha-ras oncogene expression directed by a milk protein gene promoter: tissue specificity, hormonal regulation, and tumor induction in transgenic mice. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1299–1303. doi: 10.1073/pnas.84.5.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum J. L., Zeigler M. E., Wicha M. S. Regulation of rat mammary gene expression by extracellular matrix components. Exp Cell Res. 1987 Dec;173(2):322–340. doi: 10.1016/0014-4827(87)90274-6. [DOI] [PubMed] [Google Scholar]
- Bornstein P., McKay J., Liska D. J., Apone S., Devarayalu S. Interactions between the promoter and first intron are involved in transcriptional control of alpha 1(I) collagen gene expression. Mol Cell Biol. 1988 Nov;8(11):4851–4857. doi: 10.1128/mcb.8.11.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouché J. P. The effect of spermidine on endonuclease inhibition by agarose contaminants. Anal Biochem. 1981 Jul 15;115(1):42–45. doi: 10.1016/0003-2697(81)90519-4. [DOI] [PubMed] [Google Scholar]
- Brinster R. L., Allen J. M., Behringer R. R., Gelinas R. E., Palmiter R. D. Introns increase transcriptional efficiency in transgenic mice. Proc Natl Acad Sci U S A. 1988 Feb;85(3):836–840. doi: 10.1073/pnas.85.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell S. M., Rosen J. M., Hennighausen L. G., Strech-Jurk U., Sippel A. E. Comparison of the whey acidic protein genes of the rat and mouse. Nucleic Acids Res. 1984 Nov 26;12(22):8685–8697. doi: 10.1093/nar/12.22.8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnay P., Treisman R., Mellon P., Chao M., Axel R., Maniatis T. Differences in human alpha- and beta-globin gene expression in mouse erythroleukemia cells: the role of intragenic sequences. Cell. 1984 Aug;38(1):251–263. doi: 10.1016/0092-8674(84)90547-6. [DOI] [PubMed] [Google Scholar]
- Chen L. H., Bissell M. J. A novel regulatory mechanism for whey acidic protein gene expression. Cell Regul. 1989 Nov;1(1):45–54. doi: 10.1091/mbc.1.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Crenshaw E. B., 3rd, Kalla K., Simmons D. M., Swanson L. W., Rosenfeld M. G. Cell-specific expression of the prolactin gene in transgenic mice is controlled by synergistic interactions between promoter and enhancer elements. Genes Dev. 1989 Jul;3(7):959–972. doi: 10.1101/gad.3.7.959. [DOI] [PubMed] [Google Scholar]
- Eisenstein R. S., Rosen J. M. Both cell substratum regulation and hormonal regulation of milk protein gene expression are exerted primarily at the posttranscriptional level. Mol Cell Biol. 1988 Aug;8(8):3183–3190. doi: 10.1128/mcb.8.8.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Geissler E. N., Cheng S. V., Gusella J. F., Housman D. E. Genetic analysis of the dominant white-spotting (W) region on mouse chromosome 5: identification of cloned DNA markers near W. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9635–9639. doi: 10.1073/pnas.85.24.9635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves D. R., Wilson F. D., Lang G., Kioussis D. Human CD2 3'-flanking sequences confer high-level, T cell-specific, position-independent gene expression in transgenic mice. Cell. 1989 Mar 24;56(6):979–986. doi: 10.1016/0092-8674(89)90631-4. [DOI] [PubMed] [Google Scholar]
- Grosschedl R., Baltimore D. Cell-type specificity of immunoglobulin gene expression is regulated by at least three DNA sequence elements. Cell. 1985 Jul;41(3):885–897. doi: 10.1016/s0092-8674(85)80069-6. [DOI] [PubMed] [Google Scholar]
- Grosveld F., van Assendelft G. B., Greaves D. R., Kollias G. Position-independent, high-level expression of the human beta-globin gene in transgenic mice. Cell. 1987 Dec 24;51(6):975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- Hennighausen L. G., Sippel A. E. Characterization and cloning of the mRNAs specific for the lactating mouse mammary gland. Eur J Biochem. 1982 Jun 15;125(1):131–141. doi: 10.1111/j.1432-1033.1982.tb06660.x. [DOI] [PubMed] [Google Scholar]
- Hobbs A. A., Richards D. A., Kessler D. J., Rosen J. M. Complex hormonal regulation of rat casein gene expression. J Biol Chem. 1982 Apr 10;257(7):3598–3605. [PubMed] [Google Scholar]
- Lee E. Y., Lee W. H., Kaetzel C. S., Parry G., Bissell M. J. Interaction of mouse mammary epithelial cells with collagen substrata: regulation of casein gene expression and secretion. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1419–1423. doi: 10.1073/pnas.82.5.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E. Y., Parry G., Bissell M. J. Modulation of secreted proteins of mouse mammary epithelial cells by the collagenous substrata. J Cell Biol. 1984 Jan;98(1):146–155. doi: 10.1083/jcb.98.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. F., DeMayo F. J., Atiee S. H., Rosen J. M. Tissue-specific expression of the rat beta-casein gene in transgenic mice. Nucleic Acids Res. 1988 Feb 11;16(3):1027–1041. doi: 10.1093/nar/16.3.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M. L., Aggeler J., Farson D. A., Hatier C., Hassell J., Bissell M. J. Influence of a reconstituted basement membrane and its components on casein gene expression and secretion in mouse mammary epithelial cells. Proc Natl Acad Sci U S A. 1987 Jan;84(1):136–140. doi: 10.1073/pnas.84.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUNFORD R. E. CHANGES IN THE MAMMARY GLANDS OF RATS AND MICE DURING PREGNANCY, LACTATION AND INVOLUTION. 1. HISTOLOGICAL STRUCTURE. J Endocrinol. 1963 Dec;28:1–15. doi: 10.1677/joe.0.0280001. [DOI] [PubMed] [Google Scholar]
- McKenzie R. M., Larson B. L. Purification and partial characterization of a unique group of phosphoproteins from rat milk whey. J Dairy Sci. 1978 Jun;61(6):723–728. doi: 10.3168/jds.S0022-0302(78)83639-X. [DOI] [PubMed] [Google Scholar]
- Merrill G. F., Hauschka S. D., McKnight S. L. tk Enzyme expression in differentiating muscle cells is regulated through an internal segment of the cellular tk gene. Mol Cell Biol. 1984 Sep;4(9):1777–1784. doi: 10.1128/mcb.4.9.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll C. S., Tucker H. A. Estimates of parenchymal, stromal, and lymph node deoxyribonucleic acid in mammary glands of C3H/Crgl-2 mice. Life Sci. 1965 May;4(9):993–1001. doi: 10.1016/0024-3205(65)90203-1. [DOI] [PubMed] [Google Scholar]
- Pittius C. W., Hennighausen L., Lee E., Westphal H., Nicols E., Vitale J., Gordon K. A milk protein gene promoter directs the expression of human tissue plasminogen activator cDNA to the mammary gland in transgenic mice. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5874–5878. doi: 10.1073/pnas.85.16.5874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittius C. W., Sankaran L., Topper Y. J., Hennighausen L. Comparison of the regulation of the whey acidic protein gene with that of a hybrid gene containing the whey acidic protein gene promoter in transgenic mice. Mol Endocrinol. 1988 Nov;2(11):1027–1032. doi: 10.1210/mend-2-11-1027. [DOI] [PubMed] [Google Scholar]
- Rosen J. M., Rodgers J. R., Couch C. H., Bisbee C. A., David-Inouye Y., Campbell S. M., Yu-Lee L. Y. Multihormonal regulation of milk protein gene expression. Ann N Y Acad Sci. 1986;478:63–76. doi: 10.1111/j.1749-6632.1986.tb15521.x. [DOI] [PubMed] [Google Scholar]
- Schoenenberger C. A., Andres A. C., Groner B., van der Valk M., LeMeur M., Gerlinger P. Targeted c-myc gene expression in mammary glands of transgenic mice induces mammary tumours with constitutive milk protein gene transcription. EMBO J. 1988 Jan;7(1):169–175. doi: 10.1002/j.1460-2075.1988.tb02797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons J. P., McClenaghan M., Clark A. J. Alteration of the quality of milk by expression of sheep beta-lactoglobulin in transgenic mice. Nature. 1987 Aug 6;328(6130):530–532. doi: 10.1038/328530a0. [DOI] [PubMed] [Google Scholar]
- Singh H., Sen R., Baltimore D., Sharp P. A. A nuclear factor that binds to a conserved sequence motif in transcriptional control elements of immunoglobulin genes. Nature. 1986 Jan 9;319(6049):154–158. doi: 10.1038/319154a0. [DOI] [PubMed] [Google Scholar]
- Tomasetto C., Wolf C., Rio M. C., Mehtali M., LeMeur M., Gerlinger P., Chambon P., Lathe R. Breast cancer protein PS2 synthesis in mammary gland of transgenic mice and secretion into milk. Mol Endocrinol. 1989 Oct;3(10):1579–1584. doi: 10.1210/mend-3-10-1579. [DOI] [PubMed] [Google Scholar]
- Vilotte J. L., Soulier S., Stinnakre M. G., Massoud M., Mercier J. C. Efficient tissue-specific expression of bovine alpha-lactalbumin in transgenic mice. Eur J Biochem. 1989 Dec 8;186(1-2):43–48. doi: 10.1111/j.1432-1033.1989.tb15175.x. [DOI] [PubMed] [Google Scholar]
- Wiens D., Park C. S., Stockdale F. E. Milk protein expression and ductal morphogenesis in the mammary gland in vitro: hormone-dependent and -independent phases of adipocyte-mammary epithelial cell interaction. Dev Biol. 1987 Mar;120(1):245–258. doi: 10.1016/0012-1606(87)90122-9. [DOI] [PubMed] [Google Scholar]