Abstract
Nervous system development requires the correct specification of neuron position and identity, followed by accurate neuron class-specific dendritic development and axonal wiring. Recently the dendritic arborization (DA) sensory neurons of the Drosophila larval peripheral nervous system (PNS) have become powerful genetic models in which to elucidate both general and class-specific mechanisms of neuron differentiation. There are four main DA neuron classes (I-IV)1. They are named in order of increasing dendrite arbor complexity, and have class-specific differences in the genetic control of their differentiation2-10. The DA sensory system is a practical model to investigate the molecular mechanisms behind the control of dendritic morphology11-13 because: 1) it can take advantage of the powerful genetic tools available in the fruit fly, 2) the DA neuron dendrite arbor spreads out in only 2 dimensions beneath an optically clear larval cuticle making it easy to visualize with high resolution in vivo, 3) the class-specific diversity in dendritic morphology facilitates a comparative analysis to find key elements controlling the formation of simple vs. highly branched dendritic trees, and 4) dendritic arbor stereotypical shapes of different DA neurons facilitate morphometric statistical analyses.
DA neuron activity modifies the output of a larval locomotion central pattern generator14-16. The different DA neuron classes have distinct sensory modalities, and their activation elicits different behavioral responses14,16-20. Furthermore different classes send axonal projections stereotypically into the Drosophila larval central nervous system in the ventral nerve cord (VNC)21. These projections terminate with topographic representations of both DA neuron sensory modality and the position in the body wall of the dendritic field7,22,23. Hence examination of DA axonal projections can be used to elucidate mechanisms underlying topographic mapping7,22,23, as well as the wiring of a simple circuit modulating larval locomotion14-17.
We present here a practical guide to generate and analyze genetic mosaics24 marking DA neurons via MARCM (Mosaic Analysis with a Repressible Cell Marker)1,10,25 and Flp-out22,26,27 techniques (summarized in Fig. 1).
Protocol
1.Preparation of reagents
- Prepare Ca++ -free HL3.1 saline28.
- In mM: 70 NaCl, 5 KCl, 20 MgCl2 , 10 NaHCO3 , 5 HEPES, 115 sucrose, and 5 trehalose; pH 7.2. Filter sterilize and store at 4°C. Note: Ca++ -free solution prevents muscle contraction during dissection.
- Make poly-L-lysine (PLL) coverslips.
- Dissolve 100mg PLL in 4.2ml water and make 300μl aliquots in Eppendorf tubes and freeze at -20°C.
- Before coating coverslips, first thaw an aliquot, bring it up to 10ml in ddH2O and add 20μl of Kodak Photo-Flo; the final PLL concentration is 0.7 mg/ml.
- Submerge coverslips for 30mins in PLL solution, remove and dry, then rinse briefly with water and dry. Repeat twice. Treated coverslips last approximately one month.
2. Genetic crosses
To generate MARCM clones. Here is an example cross using a pan-DA driver11,27: FRT2A x hsFLP; Gal4109(2)80, UAS-mCD8::GFP; tub-Gal80 FRT2A/SM5-TM6B
To generate Flp-out clones. Here is an example cross using a class IV-specific driver8: ppk-Gal4 x yw, hsFLP;UAS-FRT-CD2,y+-stop-FRT-mCD8::GFP
3. Collection of embryos
Keep Drosophila crosses in a collection bottle at 25°C and collect embryos on an apple juice agar plate29 spread with a thin layer of yeast paste30,31.
4. Heat shock treatment of embryos
Place an empty plate in opposition to the apple juice agar plate upon which the embryos have been laid. Seal around these two plates with Parafilm (Fig. 2).
During heat shock, submerge the plate in the water bath and hold it down using a metal weight.
- For MARCM embryos
- Collect embryos for 2h and incubate in a 10cm petri dish surrounded by moistened tissues at 25°C for an additional 2h. Note: Adjusting the heat shock protocol alters the frequency at which clones are generated.
- For a smaller number of clones, aiming for single isolated neurons, submerge and heat shock in the water bath for 1h at 38°C.
- For a larger number of clones, heat shock for 45mins at 38°C, recover at RT 30mins, then heat shock again for an additional 30mins.
Collect Flp-out embryos for 24h, and heat shock for 1h at 38°C.
5. Screening for clones
After heat shock, remove the lid of the water-tight arrangement and place the apple juice agar plate in a 10cm petri dish surrounded by moistened tissues. Culture the embryos and subsequent larvae at 25°C until wandering 3rd instar. Note: culture conditions, especially nutrition, have been shown to alter DA dendritic arbor morphology32,33. Make sure that growing larvae have access to yeast paste at all times; monitor and replenish the yeast paste as required.
From this point in the protocol onwards, manipulate larvae gently using insect forceps.
Briefly and gently rinse the larvae in tap water, and then place onto an agar plate. Note: This step reduces background auto-fluorescence from food or dirt on the larval surface.
Examine the larvae under a powerful fluorescence dissecting microscope. Identify larvae with GFP-positive neurons/cells in the body wall.
6. in vivo imaging of dendrites
Place the larva into a depression slide glass with a small drop of 80% glycerol. Place a coverslip on slide to immobilize the larva. Ensure that no air remains between the larva and the coverslip.
Gently push the coverslip to roll the larva to allow visualization of the neuron of interest. Image the mCD8::GFP-labeled dendritic arbor via confocal microscopy.
7. Larval dissection
Note before beginning: DA neuron dendrites degrade rapidly after the initiation of dissection. Dissect each individual larva in less than 5min to ensure good dendrite morphology.
- Dissect wandering 3rd instar larvae on a Sylgard plate as described previously34 with the following modifications:
- For the imaging of DA neuron axon termini, ensure that the CNS is intact and the segmental nerves are not broken. After opening the larvae, first carefully cut the tracheal connections to the body wall and then gently remove the entire gut.
- If imaging the dendrites alone, remove the CNS to allow a flatter mounting.
8. Fixation and blocking of larval fillets
With the larva still pinned to the Sylgard plate, fix it in 4% PFA in PBS for 20mins at RT on a gently rotating shaker.
Remove traces of larval tissues (fat body, trachea, etc.) after fixation.
Wash in PBST (PBS with 0.1% Triton X-100) 3x 10mins on the Sylgard plate and shaker. If examining axon termini, keep the fillet on the Sylgard plate. (If staining dendrites it is possible to transfer the larval fillets to a 0.5 ml tube at this step34)
Block the larvae for 20mins at RT in 5% normal donkey serum (NDS) in PBST on a shaker.
9. Staining of larval fillets
Remove the blocking solution and incubate in primary antibody (in 5%NDS/PBST) overnight at 4°C in a small Tupperware container surrounded by moistened tissues.
Remove the tubes from 4°C and incubate an additional hour at RT.
Wash 6x 10mins in PBST. Add the secondary antibody (in 5%NDS/PBST), and cover to prevent fluorophore photo-bleaching34.
Incubate either at RT for two hours, or overnight at 4°C followed by one hour at RT.
Wash the larvae 6x 10mins in PBST and proceed to mounting.
10. Mounting of larval fillets for examination of the dendrite arbor
Mount each larval fillet as flat as possible using dissecting scissors or a scalpel to cut off the head (including the mouth hooks), and the posterior (including the spiracles)34.
Place the larval fillet on the slide cuticle-side down, mount in 80% glycerol, and seal the sides of the coverslip with nail polish for a 'quick' mount34.
- For a permanent mounting and a clearer image
- Place the dissected larval fillet muscle-side down onto a drop of PBS on a PLL coverslip, it will quickly adhere to the coverslip.
- Remove as much liquid as possible after mounting, however do not allow it to completely dry.
- Take the coverslip (with attached larval fillet) through an ethanol series: 35% ethanol, followed by 50%, 70%, 95%, and finally 2x 100% ethanol each for 10 minutes (the 2nd 100% EtOH solution should be changed frequently); finally, wash 2-3x 10mins in Xylene.
- Put a drop of DPX mountant (Distyrene Plasticizer Xylene) on a clean slide and carefully lay the coverslip fillet-side-down on top. Keep in the dark, and wait one day for the DPX to set before imaging.
11. Mounting of larval fillets for examination of the axon termini
Keep the segmental nerves running between the PNS and the CNS intact during dissection, fixation, and staining to allow the tracing of the axons from peripheral sensory neuron cell body to the VNC.
Mount each larval fillet cuticle-side down in 80% glycerol in a depression slide. Trace the axons from the DA cell body to the VNC under the confocal microscope. Note: PNS neurons in each body wall segment project to the cognate segment of the VNC.
12. Representative Results:
Representative results are shown in figures 3-5. Fig. 3 shows the entire arbor of a class IV da neuron, captured in vivo under the confocal microscope. Fig. 4 shows a close up of part of the dendrite arbor of an immunohistochemically labeled class III neuron that has been correctly fixed to preserve morphology. The associated inset shows the degradation that can occur after an unsuccessful dissection and fixation (both stained with anti-GFP antibody). Fig. 5 shows a single class IV axon terminus in the CNS (anti-GFP, green); all class IV termini are co-stained with anti-CD2 (magenta).
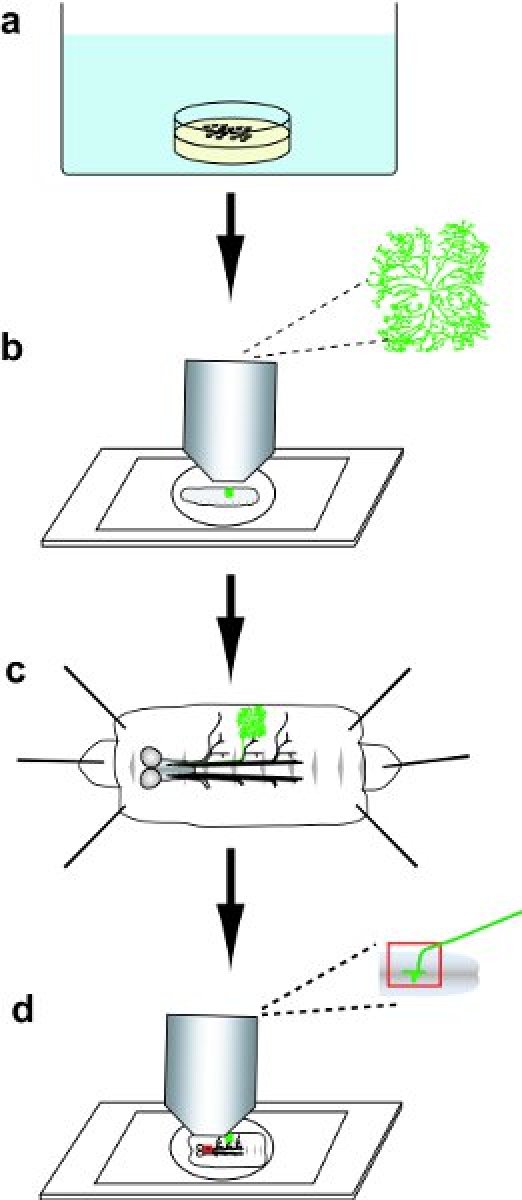 Figure 1 Protocol overview. a) Collect and then heat-shock embryos. b) Select a larva with a GFP-positive DA neuron clone, and then image the GFP-labeled dendrite arbor in the live larva. c) Dissect the larva, and then immunohistochemically stain the fillet. d) Mount the stained fillet, and then image the dendritic arbor and axonal projections of the DA neuron clones.
Figure 1 Protocol overview. a) Collect and then heat-shock embryos. b) Select a larva with a GFP-positive DA neuron clone, and then image the GFP-labeled dendrite arbor in the live larva. c) Dissect the larva, and then immunohistochemically stain the fillet. d) Mount the stained fillet, and then image the dendritic arbor and axonal projections of the DA neuron clones.
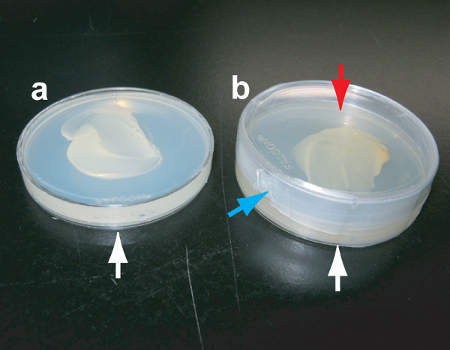 Figure 2 Preparation of the apple juice agar plate for heat shock. Take the plate (white arrow, a-b), add a second petri dish on top (red arrow b) and seal around with Parafilm (blue arrow, b).
Figure 2 Preparation of the apple juice agar plate for heat shock. Take the plate (white arrow, a-b), add a second petri dish on top (red arrow b) and seal around with Parafilm (blue arrow, b).
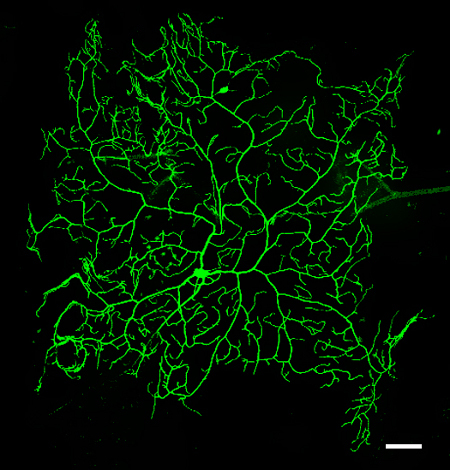 Figure 3
in vivo image of the dendrite arbor of a mCD8::GFP-labeled MARCM clone (genotype as in 2.1) representing a class IV (v'ada) neuron of a 3rd instar larva. Scale bar is 50μm.
Figure 3
in vivo image of the dendrite arbor of a mCD8::GFP-labeled MARCM clone (genotype as in 2.1) representing a class IV (v'ada) neuron of a 3rd instar larva. Scale bar is 50μm.
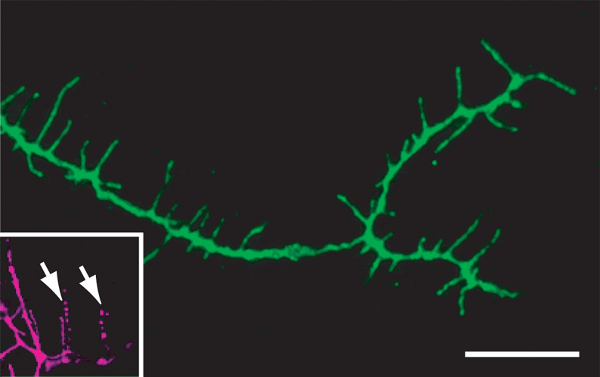 Figure 4 mCD8::GFP-labeled MARCM clone of a class III neuron (ddaA) stained with anti-GFP antibody, showing good morphology after successful dissection and fixation (green). The inset shows dendrite degradation (white arrows) occurring after unsuccessful dissection and fixation (magenta). Scale bar is 25μm.
Figure 4 mCD8::GFP-labeled MARCM clone of a class III neuron (ddaA) stained with anti-GFP antibody, showing good morphology after successful dissection and fixation (green). The inset shows dendrite degradation (white arrows) occurring after unsuccessful dissection and fixation (magenta). Scale bar is 25μm.
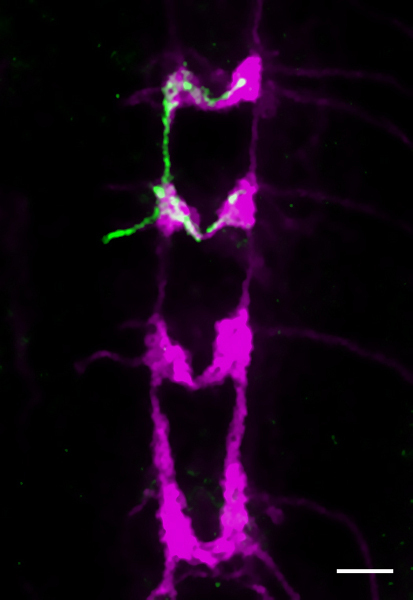 Figure 5 The 3rd instar larval VNC. A Flp-out clone of a single class VI (vdaB) axon terminus (genotype as in 2.2) is detected using anti-GFP antibody (green). Anti-CD2 labels all class IV termini (magenta). Scale bar is 25μm.
Figure 5 The 3rd instar larval VNC. A Flp-out clone of a single class VI (vdaB) axon terminus (genotype as in 2.2) is detected using anti-GFP antibody (green). Anti-CD2 labels all class IV termini (magenta). Scale bar is 25μm.
Discussion
The Drosophila larval DA neuron model provides one excellent genetic system to investigate mechanisms that control neuron morphology and circuit formation. MARCM is generally used for labeling and for generating mutant DA neuron clones. For MARCM we use either a pan-neural (e.g. Gal4c155) or DA neuron-specific driver. Using a pan-neural driver it is possible to directly use several stocks widely available from public stock centers. However using a DA-neuron specific driver can be advantageous because marked clones will not be generated in the CNS, and such clones may complicate the analysis of DA axonal termini. A list of commonly used DA-specific Gal4 lines can found in Shimono et al (2009)27. Flp-out may be particularly useful when investigators wish to concomitantly ectopically express a gene using the Gal4-UAS binary system35 and measure morphology in a single labeled neuron. In addition the imaging protocols presented here can be used when DA neurons are marked using the Gal4-UAS system35 alone.
If a good fluorescent dissecting microscope is unavailable, it is possible to select larva carrying clones using on a normal fluorescent microscope and an objective with a long working distance. During live imaging of the dendrite arbor (section 6) we immobilize the larva solely through downward force exerted by the coverslip. In adaptation of this protocol for other uses investigators can also immobilize larvae through anesthesia36,37.
During immunohistological staining, we generally use anti-GFP or anti-CD8 for labeling the neuron. In Flp-out experiments anti-CD2 can additionally mark all neurons in which the Gal4 driver line used is active (Fig. 5). When examining DA axon terminals in the VNC, anti-Fasciclin2 antibody labeling may be to used to highlight landmark axon tracts7,22,38.
When initially establishing these techniques, note that poor culture conditions can alter neuron morphology32. Furthermore, DA neuron dendrites, especially those of class III and class IV, will degrade rapidly after the beginning of dissection. We suggest dissecting and fixing larva individually. Fixation should occur within 5mins of initiating dissection to ensure good maintenance of dendrite morphology. Finally, mounting larvae as flat as possible will greatly aid subsequent morphometric analysis.
Disclosures
No conflicts of interest declared.
Acknowledgments
The authors thank RIKEN for funding. We also thank Cagri Yalgin, Caroline Delandre, and Jay Parrish for discussions on genetic and immunohistochemistry protocols.
References
- Grueber WB, Jan LY, Jan YN. Tiling of the Drosophila epidermis by multidendritic sensory neurons. Development. 2002;129:2867–2878. doi: 10.1242/dev.129.12.2867. [DOI] [PubMed] [Google Scholar]
- Crozatier M, Vincent A. Control of multidendritic neuron differentiation in Drosophila: the role of Collier. Dev Biol. 2008;315:232–242. doi: 10.1016/j.ydbio.2007.12.030. [DOI] [PubMed] [Google Scholar]
- Hattori Y, Sugimura K, Uemura T. Selective expression of Knot/Collier, a transcriptional regulator of the EBF/Olf-1 family, endows the Drosophila sensory system with neuronal class-specific elaborated dendritic patterns. Genes Cells. 2007;12:1011–1022. doi: 10.1111/j.1365-2443.2007.01107.x. [DOI] [PubMed] [Google Scholar]
- Jinushi-Nakao S. Knot/Collier and cut control different aspects of dendrite cytoskeleton and synergize to define final arbor shape. Neuron. 2007;56:963–978. doi: 10.1016/j.neuron.2007.10.031. [DOI] [PubMed] [Google Scholar]
- Sugimura K, Satoh D, Estes P, Crews S, Uemura T. Development of morphological diversity of dendrites in Drosophila by the BTB-zinc finger protein abrupt. Neuron. 2004;43:809–822. doi: 10.1016/j.neuron.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Li W, Wang F, Menut L, Gao FB. BTB/POZ-zinc finger protein abrupt suppresses dendritic branching in a neuronal subtype-specific and dosage-dependent. 2004;43:823–834. doi: 10.1016/j.neuron.2004.08.040. [DOI] [PubMed] [Google Scholar]
- Zlatic M, Landgraf M, Bate M. Genetic specification of axonal arbors: atonal regulates robo3 to position terminal branches in the Drosophila nervous system. Neuron. 2003;37:41–51. doi: 10.1016/s0896-6273(02)01131-5. [DOI] [PubMed] [Google Scholar]
- Grueber WB, Ye B, Moore AW, Jan LY, Jan YN. Dendrites of distinct classes of Drosophila sensory neurons show different capacities for homotypic repulsion. Curr Biol. 2003;13:618–626. doi: 10.1016/s0960-9822(03)00207-0. [DOI] [PubMed] [Google Scholar]
- Grueber WB, Jan LY, Jan YN. Different levels of the homeodomain protein cut regulate distinct dendrite branching patterns of Drosophila multidendritic neurons. Cell. 2003;112:805–818. doi: 10.1016/s0092-8674(03)00160-0. [DOI] [PubMed] [Google Scholar]
- Moore AW, Jan LY, Jan YN. hamlet, a binary genetic switch between single- and multiple- dendrite neuron morphology. Science. 2002;297:1355–1358. doi: 10.1126/science.1072387. [DOI] [PubMed] [Google Scholar]
- Gao FB, Brenman JE, Jan LY, Jan YN. Genes regulating dendritic outgrowth, branching, and routing in Drosophila. Genes Dev. 1999;13:2549–2561. doi: 10.1101/gad.13.19.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corty MM, Matthews BJ, Grueber WB. Molecules and mechanisms of dendrite development in Drosophila. Development. 2009;136:1049–1061. doi: 10.1242/dev.014423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AW. Intrinsic mechanisms to define neuron class-specific dendrite arbor morphology. Cell Adh. Migr. 2008;2:81–82. doi: 10.4161/cam.2.2.6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes CL, Thomas JB. A sensory feedback circuit coordinates muscle activity in Drosophila. Mol. Cell. Neurosci. 2007;35:383–396. doi: 10.1016/j.mcn.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura Y. Selection of Behaviors and Segmental Coordination During Larval Locomotion Is Disrupted by Nuclear Polyglutamine Inclusions in a New Drosophila Huntington's Disease-Like Model. J Neurogenet. 2010;24:194–206. doi: 10.3109/01677063.2010.514367. [DOI] [PubMed] [Google Scholar]
- Song W, Onishi M, Jan LY, Jan YN. Peripheral multidendritic sensory neurons are necessary for rhythmic locomotion behavior in Drosophila larvae. Proc. Natl. Acad. Sci. U. S. A. 2007;104:5199–5204. doi: 10.1073/pnas.0700895104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang RY. Nociceptive neurons protect Drosophila larvae from parasitoid wasps. Curr Biol. 2007;17:2105–2116. doi: 10.1016/j.cub.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y. Light-avoidance-mediating photoreceptors tile the Drosophila larval body wall. Nature. 2010;468:921–926. doi: 10.1038/nature09576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng LE, Song W, Looger LL, Jan LY, Jan YN. The role of the TRP channel NompC in Drosophila larval and adult locomotion. Neuron. 2010;67:373–380. doi: 10.1016/j.neuron.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock DT, Landry C, Galko MJ. Cytokine signaling mediates UV-induced nociceptive sensitization in Drosophila larvae. Curr Biol. 2009;19:799–806. doi: 10.1016/j.cub.2009.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafer N, Schedl P. Dissection of Larval CNS in Drosophila Melanogaster. J. Vis. Exp. 2006;(1):e85–e85. doi: 10.3791/85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueber WB. Projections of Drosophila multidendritic neurons in the central nervous system: links with peripheral dendrite morphology. Development. 2007;134:55–64. doi: 10.1242/dev.02666. [DOI] [PubMed] [Google Scholar]
- Merritt DJ, Whitington PM. Central projections of sensory neurons in the Drosophila embryo correlate with sensory modality, soma position, and proneural gene function. J Neurosci. 1995;15:1755–1767. doi: 10.1523/JNEUROSCI.15-03-01755.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair SS. Genetic mosaic techniques for studying Drosophila development. Development. 2003;130:5065–5072. doi: 10.1242/dev.00774. [DOI] [PubMed] [Google Scholar]
- Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- Wong AM, Wang JW, Axel R. Spatial representation of the glomerular map in the Drosophila protocerebrum. Cell. 2002;109:229–241. doi: 10.1016/s0092-8674(02)00707-9. [DOI] [PubMed] [Google Scholar]
- Shimono K. Multidendritic sensory neurons in the adult Drosophila abdomen: origins, dendritic morphology, and segment- and age-dependent programmed cell death. Neural Dev. 2009;4:37–37. doi: 10.1186/1749-8104-4-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Ueda A, Wu CF. A modified minimal hemolymph-like solution, HL3.1, for physiological recordings at the neuromuscular junctions of normal and mutant Drosophila larvae. J Neurogenet. 2004;18:377–402. doi: 10.1080/01677060490894522. [DOI] [PubMed] [Google Scholar]
- Sullivan W, Ashburner M, Hawley RS. Drosophila Protocols. Cold Spring Harbor Laboratory Press; 2000. [Google Scholar]
- Kaczynski TJ, Gunawardena S. Visualization of the Embryonic Nervous System in Whole-mount Drosophila Embryos. J. Vis. Exp. 2010;(46):e2150–e2150. doi: 10.3791/2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Featherstone DE, Chen K, Broadie K. Harvesting and preparing Drosophila embryos for electrophysiological recording and other procedures. J Vis Exp. 2009 doi: 10.3791/1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina PM, Swick LL, Andersen R, Blalock Z, Brenman JE. A novel forward genetic screen for identifying mutations affecting larval neuronal dendrite development in Drosophila melanogaster. Genetics. 2006;172:2325–2335. doi: 10.1534/genetics.105.051276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirouse V, Swick LL, Kazgan N, St Johnston D, Brenman JE. LKB1 and AMPK maintain epithelial cell polarity under energetic stress. J Cell Biol. 2007;177:387–392. doi: 10.1083/jcb.200702053. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Brent J, Werner K, McCabe BD. Drosophila Larval NMJ Immunohistochemistry. J. Vis. Exp. 2009;25:e1108–e1108. doi: 10.3791/1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Sugimura K. Distinct developmental modes and lesion-induced reactions of dendrites of two classes of Drosophila sensory neurons. J Neurosci. 2003;23:3752–3760. doi: 10.1523/JNEUROSCI.23-09-03752.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zito K, Parnas D, Fetter RD, Isacoff EY, Goodman CS. Watching a synapse grow: noninvasive confocal imaging of synaptic growth in Drosophila. Neuron. 1999;22:719–729. doi: 10.1016/s0896-6273(00)80731-x. [DOI] [PubMed] [Google Scholar]
- Landgraf M, Sanchez-Soriano N, Technau GM, Urban J, Prokop A. Charting the Drosophila neuropile: a strategy for the standardised characterisation of genetically amenable neurites. Dev Biol. 2003;260:207–225. doi: 10.1016/s0012-1606(03)00215-x. [DOI] [PubMed] [Google Scholar]


