Abstract
The corticospinal (CS) tract is the anatomical support of the exquisite motor ability to skillfully manipulate small objects, a prerogative mainly of primates1. In case of lesion affecting the CS projection system at its origin (lesion of motor cortical areas) or along its trajectory (cervical cord lesion), there is a dramatic loss of manual dexterity (hand paralysis), as seen in some tetraplegic or hemiplegic patients. Although there is some spontaneous functional recovery after such lesion, it remains very limited in the adult. Various therapeutic strategies are presently proposed (e.g. cell therapy, neutralization of inhibitory axonal growth molecules, application of growth factors, etc), which are mostly developed in rodents. However, before clinical application, it is often recommended to test the feasibility, efficacy, and security of the treatment in non-human primates. This is especially true when the goal is to restore manual dexterity after a lesion of the central nervous system, as the organization of the motor system of rodents is different from that of primates1,2. Macaque monkeys are illustrated here as a suitable behavioral model to quantify manual dexterity in primates, to reflect the deficits resulting from lesion of the motor cortex or cervical cord for instance, measure the extent of spontaneous functional recovery and, when a treatment is applied, evaluate how much it can enhance the functional recovery.
The behavioral assessment of manual dexterity is based on four distinct, complementary, reach and grasp manual tasks (use of precision grip to grasp pellets), requiring an initial training of adult macaque monkeys. The preparation of the animals is demonstrated, as well as the positioning with respect to the behavioral set-up. The performance of a typical monkey is illustrated for each task. The collection and analysis of relevant parameters reflecting precise hand manipulation, as well as the control of force, are explained and demonstrated with representative results. These data are placed then in a broader context, showing how the behavioral data can be exploited to investigate the impact of a spinal cord lesion or of a lesion of the motor cortex and to what extent a treatment may enhance the spontaneous functional recovery, by comparing different groups of monkeys (treated versus sham treated for instance). Advantages and limitations of the behavioral tests are discussed. The present behavioral approach is in line with previous reports emphasizing the pertinence of the non-human primate model in the context of nervous system diseases2,3.
Keywords: Neuroscience, Issue 57, monkey, hand, spinal cord lesion, cerebral cortex lesion, functional recovery
Protocol
The overall scheme of the experiment is depicted in Figure 1.
1. Animal preparation and transfer to the behavioral laboratory
In the laboratory, prepare the behavioral set-up: fill the wells of the different test boards (tests 1 to 3 below) with the pellets, which serve as reward during the behavioral tests.
Transfer the monkey from the group housing room into a transfer cage. The monkey is trained to enter a tunnel giving access to the primate chair, with subsequent positioning of the head. The monkey's weight is measured, before transfer in the primate chair to the laboratory.
2. Test 1: Modified Brinkman board
This test, modified and adapted from previous reports4,5, is the basic behavioral task of reference, to be conducted on every behavioral session. Initiate the video recording with the digital camera above the set-up (possibility also to place 2 additional cameras, one on each side of the board) and place the monkey in front of the Brinkman board.
Open for instance the right window on the primate chair to give access to the right hand. Using the right hand, the monkey retrieves the food pellets from the 50 slots (25 vertical and 25 horizontal).
After completion of the test, close the right window and refill the board with pellets.
Open the left window and repeat the test for the left hand.
Reward the animal at the end of the test with a few dried raisins or an almond, a procedure to be repeated at the end of each test to maintain the motivation during the whole daily session.
Additional information: Three digital video cameras are used to record the sequence for off-line processing, placed one above the board and one on each side of the board (to precisely assess the position of the fingers while performing the grasping). Within the same daily session, the monkey can perform another task (either task 2, and/or task 3 and/or task 4; to be distributed among the different days of the week). For the test 1, if the monkey started with the right hand on day 1, start with the left hand on day 2 and so on.
Optional: While the test performed above with one or the other hand separately allows comparing the performance of the left hand to the right hand (to identify the "dominant hand"), it is also possible at an early stage of the training to let the monkey perform the task with both hands simultaneously. If one hand is used more often than the other to grasp pellets, then it may be considered as the "preferred hand".
3. Test 2: Brinkman box (with and without visual control)
The Brinkman box comprises 20 wells (10 vertical and 10 horizontal). As compared to test 1, the monkey has to control the hand in a limited space, with reduced degrees of freedom to perform the precision grip movement. Fill the board with pellets and close the upper facet of the box, in order to conduct first the test in absence of visual control (relying on tactile exploration).
Place the monkey in front of the Brinkman box. Open the left window of the primate chair to test the left hand in absence of visual control. The monkey tries to retrieve the 20 pellets, while the sequence is recorded from a digital camera placed below the box.
Close the left window of the primate chair. Refill the board with pellets. Open the right window of the primate chair. The monkey repeats the test with the right hand in absence of visual control. Close the right window of the primate chair.
To test the ability to grasp pellets in the Brinkman box under visual control,open the top facet of the box.
Refill the box with pellets and the monkey performs the test using the right hand.
Close the right window of the primate chair. Refill the box with pellets.
Open the left window of the primate chair. The monkey performs the test using the left hand.Repeat step 2.5.
4. Test 3: Rotating Brinkman board
This test is comparable to the Brinkman board task (test 1), except that the board is rotating, forcing the monkey to anticipate the displacement of the board in one (clockwise) or the other (counter-clockwise) direction. Fill the board with pellets. The sequence is recorded with a digital camera placed above the set-up (possibility also to place 2 additional cameras, one on each side).
Open the right window of the primate chair. The monkey retrieves the pellets from the 32 wells, distributed on four concentric rows, while the board is turning clockwise.
Close the right window. Refill the board with pellets.
Open the left window of the primate chair. The monkey performs the test as in 4.2 with the left hand.
Repeat points 4.2 to 4.4, while the board is turning counterclockwise (one hand after the other). Repeat step 2.5.
5. Test 4: Reach and grasp drawer task
To combine prehension ability with the capacity to generate force, this test (derived from previous versions6-11) was designed so that the monkey has to open a drawer by exerting first a grip force on the knob of the drawer, followed by a load force to open the drawer, giving access to a pellet placed inside the drawer. The pellet is retrieved with the same hand while the drawer remains open, again using the precision grip. A digital camera is placed on top of the drawer, to record the trials for off-line control of the data (e.g. detection of erroneous trials).
Open the right window of the primate chair. The monkey performs 10 trials at each of the 5 different levels of resistance, using the right hand (to have at least 5 correct trials for each resistance level).
Repeat the test (50 trials) with the left hand. Repeat step 2.5.
Additional information: On the next behavioral daily session, alternate the hand with which the animal did the test first on the previous session.
6. End of behavioral session
After completion of the tests foreseen on that day, feed and reward the monkey with food in addition to the pellets received during the tests. Typically, the monkey receives cereals and fruits.
The monkey is returned to the group housing room with the mates.
The precise temporal sequence of the various tests performed is written on the protocol form.
7. Representative Results
The four behavioral tests illustrated above (Figure 1) have been used extensively in our laboratory in the context of studies aimed at investigating the functional recovery from lesion of the cervical spinal cord (Figure 2A) or of the motor cortex (Figure 2B), in absence or in presence of a treatment applied to enhance the spontaneous recovery12-19.
For test 1 (modified Brinkman board), the analysis is focused on two parameters (Figures 3 and 4A): i) the score, given by the number of pellets retrieved by the monkey in the first 30 seconds, counted separately for the vertical slots and the horizontal slots (by replaying off-line the recorded video sequence); ii) the contact time (CT), defined as the time (duration) of contact between the fingers and the pellet (see also Figure 6, bottom series of pictures). It is the time interval between the insertion of the first finger (usually the index finger) into the slot to touch the pellet and the onset of retrieval of the pellet out of the well. The time interval is measured by replaying frame by frame the video sequence. The CT is measured for the first five vertical slots and the first five horizontal slots aimed by the monkey16-18. The graphs of the score illustrate the initial training phase, the pre-lesion plateau, the dramatic drop of score (usually to zero) immediately after the lesion, the progressive (spontaneous) functional recovery towards the post-lesion plateau. The functional recovery is expressed in % by the ratio of the median post-lesion score at plateau divided by the median pre-lesion score (plateau) *100 (Figures 3 and 4A). For the CT, as an increase reflects a deficit, the functional recovery expressed in % is the ratio of the median pre-lesion CT (plateau) divided by the median post-lesion CT at plateau *100. The effect of treatments post-lesion, demonstrated based on the test 1, are illustrated in detail in previous reports from this laboratory14,15,17. A further analysis may address the issue of the strategy, namely the temporal sequence of slots visited by the monkey (Figure 4B).
For test 2 (Brinkman box), although the score can also be established as in test 1, a more meaningful parameter is the "total time", defined as the time interval between the picking of the pellet in the first slot and the picking of the pellet in the last (20th) slot (Figure 5). A functional recovery can be computed and expressed in %. It is calculated using the median total time pre-lesion (plateau after training phase) divided by the median total time post-lesion at plateau *100.
For test 3 (rotating Brinkman board), although the score can also be established as above (test 1), a sensitive parameter is the contact time (CT, defined as above in test 1), measured for the first ten slots (Figure 6).
For test 4 (.reach and grasp drawer task), the set-up comprises several detectors, recording discrete events such as trial initiation (the hand interrupts a light beam placed in front of the panel), hand touching the knob, onset of drawer pulling, end of drawer opening, hand entering the slot (pick-in time), hand withdrawal from the slot (pick-out time). Force transducers on the knob allow measuring the grip force (exerted by the thumb and index finger pressing on the knob) and the load force (exerted to pull the drawer, to counteract different levels of resistance imposed on the drawer opening). The different levels of resistance opposing the opening of the drawer were obtained by changing the intensity of current applied to a rotative electromagnetic motor attached to the back of the drawer. The set-up is designed to apply a resistive force in parallel to the orientation of the opening of the drawer. Detailed scheme of the drawer set-up is available on request to the corresponding author. All these data are collected with the interface and software Spike 2 and displayed as illustrated in Figure 7.
As previously reported14,15, these tasks represent the behavioral basis to investigate whether the spontaneous recovery from a lesion of the cervical cord may be enhanced with a specific treatment aimed at promoting axonal regeneration (Figure 8).
The retrieval score and the contact time parameters reflect different components of the manual dexterity: the first one includes the entire motor sequence (reaching, grasping, withdrawal of the hand, transport of the pellet to the mouth), whereas the second one is focused on the grasping phase only. These two parameters are particularly accurate and complementary for the modified and the rotating Brinkman board tasks. Whereas the score represents largely redundant information in these two tasks, the contact time in contrast provides more task-specific information due to the variability of the slots' positions in the rotating Brinkman board task, as compared to their static position in the modified Brinkman board task. The Brinkman box task differs from the two above mentioned tasks, as the degrees of freedom of movements with the hand are limited by the closed space. As a consequence, the positions of the different slots on the board within the Brinkman box play an important role in terms of difficulty to perform the manual prehension, due to the exiguity of the box. For instance, the grasping with the hand from the slots located on the right side of the box interferes with right lateral wall.
Consequently, the assessment of retrieval score limited to 30 seconds as in the modified Brinkman board would be biased depending on the position of the slots actually visited by the monkey during this restricted time period, generating a substantial variability from one session to another. For this reason, it is more appropriate to involve all slots (n=20) and therefore the parameter total time was chosen. In the modified Brinkman board, the total time was not considered, as the monkey may in some case loose motivation (for instance post-lesion) due to the large number of slots to be performed (n=50). Along the same line, the analysis of the contact time would implicate to take all the slots of the Brinkman box into consideration (whereas only the first five slots in the modified Brinkman board were considered as the position of the selected slots has little, if no impact on this parameter). Therefore, for the Brinkman box, we recommend in a first approach to determine the total time, as it was observed to be a highly pertinent and informational parameter, at least in our studies with monkeys subjected to a motor cortex lesion (no data available for spinal cord lesioned monkeys). Nevertheless, the contact time may be considered for the Brinkman box, but in a second step including all twenty slots, separately however for the horizontal and vertical slots (not shown).
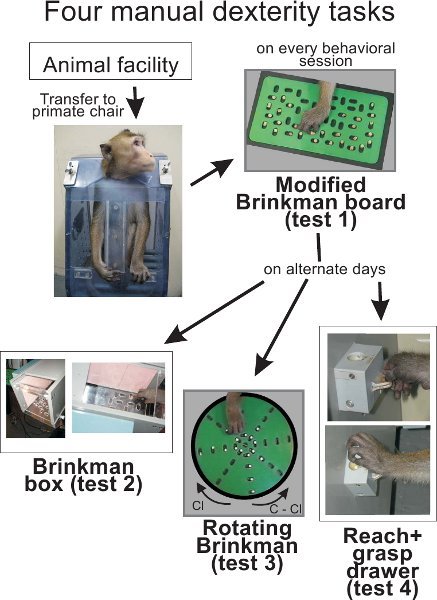 Figure 1. Overall scheme of the experiment. The animal is transferred from the animal facility into the primate chair, transported then to the behavioral laboratory. On each daily session, the monkey performs the test 1. On alternate days, the monkey then performs test 2, and/or test 3, and/or test 4. Some monkeys (especially motivated) may perform all tests on the same daily session. Food pellets (used as reward) were made of dried banana or glucose powder that is compressed in a round shape of about 4 mm in diameter. In all our behavioral tests, we use dustless precision pellets (45 mg) provided by BioServ, One 8th street, Suite One, Frenchtown, NJ 08825, USA.
Figure 1. Overall scheme of the experiment. The animal is transferred from the animal facility into the primate chair, transported then to the behavioral laboratory. On each daily session, the monkey performs the test 1. On alternate days, the monkey then performs test 2, and/or test 3, and/or test 4. Some monkeys (especially motivated) may perform all tests on the same daily session. Food pellets (used as reward) were made of dried banana or glucose powder that is compressed in a round shape of about 4 mm in diameter. In all our behavioral tests, we use dustless precision pellets (45 mg) provided by BioServ, One 8th street, Suite One, Frenchtown, NJ 08825, USA.
The dimension of the modified Brinkman board is 240mm long and 140 mm wide, whereas the dimension of the slots is 15 mm long, 8 mm wide and 6 mm deep. The diameter of the board in the rotative Brinkman board is 114 mm.
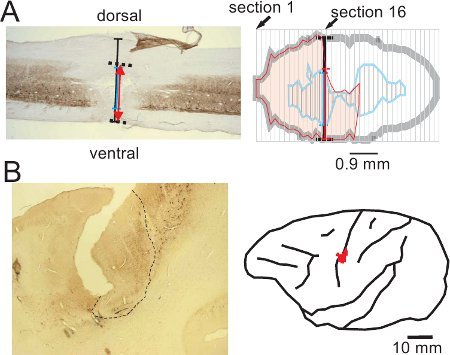 Figure 2. Representative (surgical) lesion of the cervical cord (panel A; modified from15) and (chemical) lesion of the motor cortex (panel B; modified from16), derived from corresponding histological sections, processed for SMI-32 staining. The maximal extent of the cervical cord lesion has been reconstructed from consecutive sagittal sections of the spinal cord (panel A), whereas the extent and position of the motor cortex lesion has been reconstructed from consecutive frontal sections of the brain and re-positioned on a lateral view of the corresponding brain hemisphere (red spot in panel B). The cervical cord lesion resulted from a transection with a surgery blade at the level C7-C8, resulting in a sub-hemisection interrupting unilaterally the main CS tract component in the dorsolateral funiculus, above the motoneurons controlling hand muscles14,15,20. The permanent cortical lesion was produced by infusion of ibotenic acid13,16,17, at sites covering the hand representation previously established using intracortical microstimulation (ICMS, see16,21,22). The cortical lesion territory appears as an abrupt interruption of the SMI-32 staining of neurons in layers III and V in the rostral bank of the central sulcus (area delineated with the dashed line in panel B).
Figure 2. Representative (surgical) lesion of the cervical cord (panel A; modified from15) and (chemical) lesion of the motor cortex (panel B; modified from16), derived from corresponding histological sections, processed for SMI-32 staining. The maximal extent of the cervical cord lesion has been reconstructed from consecutive sagittal sections of the spinal cord (panel A), whereas the extent and position of the motor cortex lesion has been reconstructed from consecutive frontal sections of the brain and re-positioned on a lateral view of the corresponding brain hemisphere (red spot in panel B). The cervical cord lesion resulted from a transection with a surgery blade at the level C7-C8, resulting in a sub-hemisection interrupting unilaterally the main CS tract component in the dorsolateral funiculus, above the motoneurons controlling hand muscles14,15,20. The permanent cortical lesion was produced by infusion of ibotenic acid13,16,17, at sites covering the hand representation previously established using intracortical microstimulation (ICMS, see16,21,22). The cortical lesion territory appears as an abrupt interruption of the SMI-32 staining of neurons in layers III and V in the rostral bank of the central sulcus (area delineated with the dashed line in panel B).
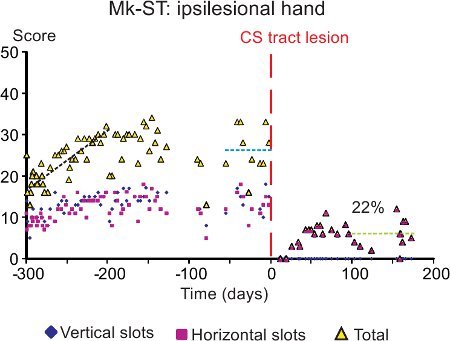 Figure 3. Representative data derived from the modified Brinkman board task (test 1) performed by a monkey subjected to a lesion of the spinal cord (as illustrated in Figure 2A). The graph shows the score (ordinate), separately for the vertical slots (blue symbols) and the horizontal slots (red symbols). The yellow symbols are for the sum of vertical and horizontal scores on a given daily session. In the abscissa, the time is for the consecutive days of the behavioral sessions. The vertical dashed redline (day 0) is the day at which the lesion was performed. Three different periods are highlighted: the first one (black dashed line) corresponds to the training period, the second one (blue dashed line) to the plateau of performance before the lesion, and the third one (green dashed line) to the plateau of recovered performance. Data modified from15.
Figure 3. Representative data derived from the modified Brinkman board task (test 1) performed by a monkey subjected to a lesion of the spinal cord (as illustrated in Figure 2A). The graph shows the score (ordinate), separately for the vertical slots (blue symbols) and the horizontal slots (red symbols). The yellow symbols are for the sum of vertical and horizontal scores on a given daily session. In the abscissa, the time is for the consecutive days of the behavioral sessions. The vertical dashed redline (day 0) is the day at which the lesion was performed. Three different periods are highlighted: the first one (black dashed line) corresponds to the training period, the second one (blue dashed line) to the plateau of performance before the lesion, and the third one (green dashed line) to the plateau of recovered performance. Data modified from15.
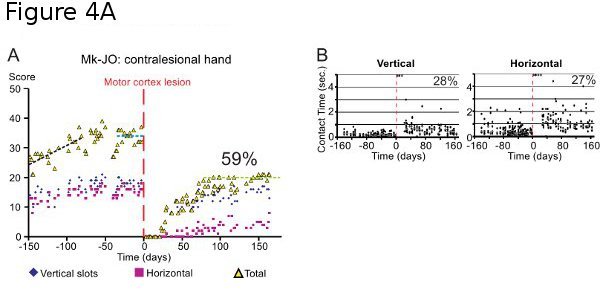
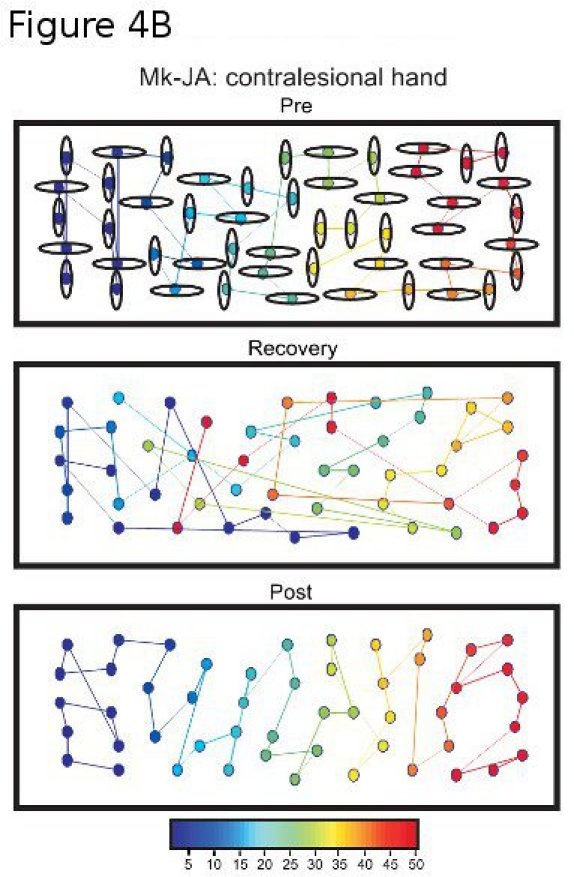 Figure 4A. Same as in Figure 3 (test 1), but in a monkey subjected to a lesion of the motor cortex (as illustrated in Figure 2B). The graphs show the score (panel A; same conventions as in Figure 3) and the contact time (CT; panel B) data. In the abscissa, the time is for the consecutive days of the behavioral sessions. The vertical dashed redline (day 0) is the day at which the lesion was performed. In panel B, each dot corresponds to the time of contact between the finger and the pellet in one slot (5 trials per orientation for each session; the grey bar represents the median value). Note that for the trials in which the animal could not perform the task (immediately after the lesion), the CT appears as a saturated value at 5 seconds. Data modified from16,17.
Figure 4A. Same as in Figure 3 (test 1), but in a monkey subjected to a lesion of the motor cortex (as illustrated in Figure 2B). The graphs show the score (panel A; same conventions as in Figure 3) and the contact time (CT; panel B) data. In the abscissa, the time is for the consecutive days of the behavioral sessions. The vertical dashed redline (day 0) is the day at which the lesion was performed. In panel B, each dot corresponds to the time of contact between the finger and the pellet in one slot (5 trials per orientation for each session; the grey bar represents the median value). Note that for the trials in which the animal could not perform the task (immediately after the lesion), the CT appears as a saturated value at 5 seconds. Data modified from16,17.
Figure 4B. Analysis of strategy adopted in the modified Brinkman board task performed with the contralesional hand, before lesion of the motor cortex (Pre), during the recovery phase (Recovery) and post-lesion at plateau (Post). The color of each slot indicates the sequential order of the slots visited by the monkey in one session (the first slot visited is depicted by the darkest blue and the last slot visited by the darkest red). Note that pre-lesion, the monkey started on the left side of the board and scanned systematically towards right. During the recovery, the sequential order was changed. At plateau post-lesion, the strategy adopted pre-lesion re-appeared (systematic scan from left to right).
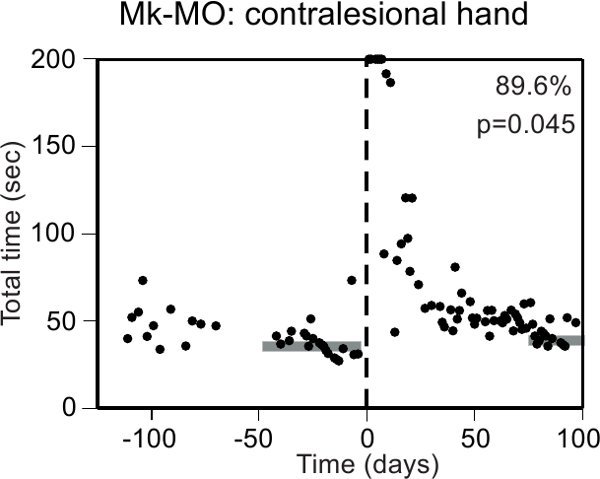 Figure 5. Representative data derived from the Brinkman box (test 2), for a monkey performing the task under visual control. The ordinate is the total time needed to empty the 20 wells along the daily sessions (abscissa), conducted before and after a lesion of the motor cortex (vertical dashed line). The dimensions of the accessible volume within the box are 1360 cm3 (120mm*110mm*103mm). Note an initial phase of training, characterized by a larger variability of the total time from one session to the next. Immediately after the cortical lesion, the monkey was not able to perform the task (data points saturated at 200 seconds). The p value is statistically significant for the difference between the median total time pre-lesion (middle of the horizontal gray rectangle at the left) and the median total time post-lesion in the last sessions (middle of the horizontal gray rectangle at the right). The percentage of functional recovery is 89.6% whereas the volume of the motor cortex lesion was 41.8 mm3. Data modified from19.
Figure 5. Representative data derived from the Brinkman box (test 2), for a monkey performing the task under visual control. The ordinate is the total time needed to empty the 20 wells along the daily sessions (abscissa), conducted before and after a lesion of the motor cortex (vertical dashed line). The dimensions of the accessible volume within the box are 1360 cm3 (120mm*110mm*103mm). Note an initial phase of training, characterized by a larger variability of the total time from one session to the next. Immediately after the cortical lesion, the monkey was not able to perform the task (data points saturated at 200 seconds). The p value is statistically significant for the difference between the median total time pre-lesion (middle of the horizontal gray rectangle at the left) and the median total time post-lesion in the last sessions (middle of the horizontal gray rectangle at the right). The percentage of functional recovery is 89.6% whereas the volume of the motor cortex lesion was 41.8 mm3. Data modified from19.
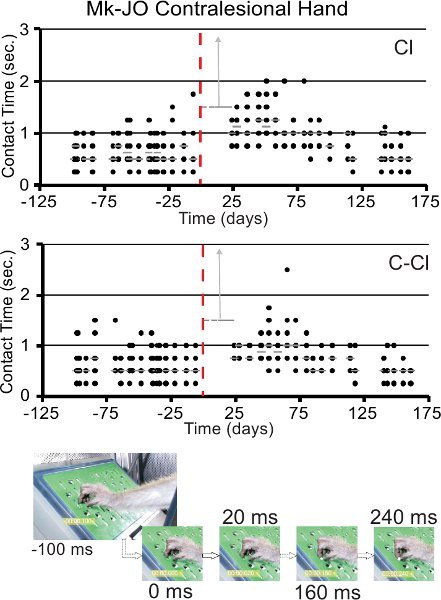 Figure 6. Representative results (top two graphs) derived from the rotative Brinkman board task (test 3), with illustration of the contact time measured pre-lesion and post-lesion, with the same conventions as in Figure 4A (panel B), for a monkey subjected to a lesion of the motor cortex (data modified from17). The top graph is for a clockwise ("Cl") rotation of the board, whereas the bottom graph is for a counterclockwise ("C-Cl") rotation of the board. The two vertical gray arrows indicate that the contact time was infinitely long in few sessions immediately after the lesion, as the monkey was unable to perform the task with the contralesional hand. The series of pictures at the bottom of the figure illustrate the method to measure the contact time (valid for both the modified Brinkman board and the rotating Brinkman board). The leftmost picture shows the hand approaching the slot containing the pellet (100 ms before contact between the index finger and the pellet). The next frame on the right corresponds to the time point of contact (0 ms). Then the contact time is defined as the time interval (in ms) running until reaching the frame (rightmost one) corresponding to the time point at which the pellet is taken out of the slot. The contact time here is 240 ms.
Figure 6. Representative results (top two graphs) derived from the rotative Brinkman board task (test 3), with illustration of the contact time measured pre-lesion and post-lesion, with the same conventions as in Figure 4A (panel B), for a monkey subjected to a lesion of the motor cortex (data modified from17). The top graph is for a clockwise ("Cl") rotation of the board, whereas the bottom graph is for a counterclockwise ("C-Cl") rotation of the board. The two vertical gray arrows indicate that the contact time was infinitely long in few sessions immediately after the lesion, as the monkey was unable to perform the task with the contralesional hand. The series of pictures at the bottom of the figure illustrate the method to measure the contact time (valid for both the modified Brinkman board and the rotating Brinkman board). The leftmost picture shows the hand approaching the slot containing the pellet (100 ms before contact between the index finger and the pellet). The next frame on the right corresponds to the time point of contact (0 ms). Then the contact time is defined as the time interval (in ms) running until reaching the frame (rightmost one) corresponding to the time point at which the pellet is taken out of the slot. The contact time here is 240 ms.
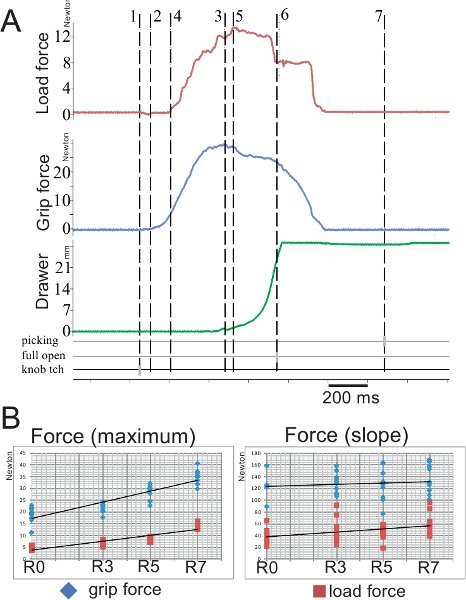 Figure 7. Representative data derived from a session performed by one monkey on the reach and grasp drawer task (test 4).
Figure 7. Representative data derived from a session performed by one monkey on the reach and grasp drawer task (test 4).
Panel A: Raw data corresponding to three parameters acquired online during a single trial: load force in red, grip force in blue and displacement of the drawer in green. Several markers were also acquired during the task: picking time corresponds to the time of the reward grasping, full open to the full opening of the drawer and knob touch (tch) to the time point when the animal first touches the knob. For the analysis, seven cursors were placed at critical time points in the unfolding task (e.g. 3 gray cursors on the bottom three horizontal lines): 1) time locked to the touch of the knob by the animal; 2) onset of grip force; 3) maximal grip force; 4) onset of load force; 5) maximal load force; 6) time locked when the drawer is fully open; 7) time locked to the picking time.
Panel B: Representation of the quantitative results for two parameters recorded during the task: the grip force (force used to grasp the knob between the index finger and the thumb) and the load force (force used to open the drawer) in two diagrams: the maximal value (left graph) and the slope value (from the onset to the max.; right graph).
Four out of the five different relative levels of resistance have been illustrated here: R0 (0 Newton), R3 (1.4 N), R5 (2.75 N) to R7 (5 N). The knob of the drawer has a triangular and flat shape. The base of the triangle attached to the drawer measures 20mm and the top (15 mm from the base) consists in a circular contour of 7 mm of diameter. The drawer itself has the following dimensions: length=50 mm; width=27 mm and height= 45mm.
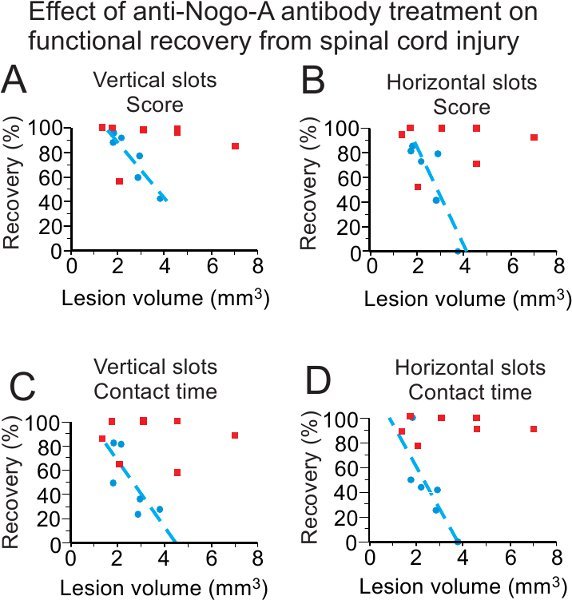
Figure 8. Reminder (modified from15) of the use of the behavioral test 1 to investigate the possible effect of a treatment (anti-Nogo-A antibody) on the functional recovery from cervical cord lesion. For both scores and contact time, as well as for both slot orientations, the group of control antibody treated monkeys (blue symbols; n=6) recovers manual dexterity less well than the group of anti-Nogo-A antibody treated monkeys (red symbols; n=7), especially for large volumes of lesion. The 2 groups differ significantly with p=0.035 (panel A), p=0.022 (panel B), p=0.035 (panel C) and p=0.008 (panel D).
Discussion
Although the present behavioral tasks have been considered so far in our laboratory in the context of studies related to lesion of the cervical cord or to lesion of the motor cortex with the aim to test various treatments (see14,15,17 and http://www.unifr.ch/neuro/rouiller >select "research" in the top bar menu, then > "motor system" > "recovery after lesion"), they may also have a broader application, as manual dexterity is also an aspect to consider in other pathologies, such as Parkinson disease (MPTP monkeys) or in case of sensory de-afferentation affecting proprioception and/or the sense of touch (especially the test 2 in absence of visual control).
The behavioral tests proposed here are suitable to investigate the motor control of distal movements of the forelimb, as involved in manual dexterity. The specificity of the tests is demonstrated by the absence of deficit (except for a couple of days) in case of a lesion which does not impair relevant components of the control system: indeed, in case of a lesion placed more caudal than the motoneurons controlling hand muscles, there was no deficit. The pertinence of the test 1 can be appreciated by comparing on video sequences taken at two time points of the post-lesion recovery curve, which differ by an enhancement of functional recovery of 25%, possibly in relation to a cell therapy treatment17.
In spite of some initial, relatively short training phase at beginning (lasting generally 2-3 months), the behavioral tests proposed here are relatively "natural" and straightforward, as compared to complex (e.g. conditional) tasks for which the training of the monkey may take nearly a year or more. The positive reinforcement is based on solid food, which is less sensitive on the ethical point of view than water deprivation, usually used in more complex tasks23. There is no need to deprive the monkeys from food to obtain stable and consistent results. The pellets received during the tasks represent the first access to the food on the day of the behavioral session (assuming that the monkey does not eat during the preceding night; however additional food may be given until the end of the afternoon of the preceding day). It is crucial that each monkey performs the behavioral session at the same time of the day, as well as respecting the same sequential order between the different monkeys forming a group in the housing room. As the monkeys are sensitive to external disturbing events, the behavioral tasks should be conducted in presence of background music, masking potential disturbing noise coming from neighboring rooms or laboratories. It is crucial that during the whole duration of the experiment (from initial training up to the last daily experimental session, a period of several months if not years), a given monkey is placed daily under the supervision of the same experimenter.
The present behavioral tests, used since several years in our laboratory to quantify manual dexterity, are to some extent comparable to other tests of manual dexterity recently reported in the literature24-28. There is however a crucial need to standardize tests across different laboratories (for better comparison), which is a tentative goal of the present report. On demand, the detailed properties of the set-ups illustrated here for tests 1-4 can be provided by the corresponding author, in order to replicate them. Beyond the issue of regenerative medicine (recovery from lesion of spinal cord or cerebral cortex), the present palette of tests may be suitable to address in normal non-human primates developmental issues (e.g. time course of motor development of dexterous movements), to investigate lateralization aspects (hand preference/dominance) and to decipher evolutionary questions by comparing the motor abilities of different species of primates, including human subjects. Note however that the dimensions of the apparatuses should be adapted according to the digits' size (thickness and length) of the primate species, as it may influence the task performance. In the present study, the tests were conducted on macaca fascicularis monkeys, ranging from 2.5 to 8 years old and weighing between 2.5 and 8 kg. The length of the index finger (used first to manipulate the pellets) ranges from 32 to 35 mm, whereas the circumference of the distal phalanx (tip) of the index finger was between 22 and 25 mm in the monkeys included in our studies. As tested in previous experiments, the same grasping tests are suitable for macaca mulatta as well.
All experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (1996) and approved by local (Swiss) veterinary authorities. All experimental procedures on the monkeys, as well as the detention conditions in the animal facility, were described in detail in recent reports from our laboratory: see references 12-18.
Disclosures
No conflicts of interest declared.
Acknowledgments
The authors would like to thank Prof. M.E. Schwab, Dr. P. Freund, Dr. A. Wyss, Dr. S. Bashir, Dr. A. Mir, Dr. J. Bloch, Dr. J.F. Brunet, Dr. J. Aebischer, Dr. A. Meszaros, Dr. V. Goetschman for their contribution to previous experiments and analyses. The experimental set-ups were constructed by André Gaillard, Bernard Aebischer and Laurent Monney. In the animal facility, the monkeys were placed under the supervision of professional animal caretakers: Josef Corpataux, Laurent Bossy and Jacques Maillard. The behavioral tests and analysis of the data, as well as histology, were conducted with the highly valuable contributions of laboratory technicians: Véronique Moret (also web master), Françoise Tinguely, Christine Roulin, Monica Bennefeld, Christiane Marti and Georgette Fischer. This work was supported by the Swiss National Science Foundation, grants No 31-61857.00, 310000-110005, 31003A-132465 (EMR), 310030-118357, 31003A-104061 (TW), 310030-120411 (ABS), PZ00P3_121646 (ES), Novartis Foundation; The National Centre of Competence in Research (NCCR) on "Neural plasticity and repair".
References
- Lemon RN. Descending pathways in motor control. Annu. Rev. Neurosci. 2008;31:195–218. doi: 10.1146/annurev.neuro.31.060407.125547. [DOI] [PubMed] [Google Scholar]
- Courtine G. Can experiments in nonhuman primates expedite the translation of treatments for spinal cord injury in humans? Nat. Med. 2007;13:561–566. doi: 10.1038/nm1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitanio JP, Emborg ME. Contributions of non-human primates to neuroscience research. Lancet. 2008;371:1126–1135. doi: 10.1016/S0140-6736(08)60489-4. [DOI] [PubMed] [Google Scholar]
- Brinkman C. Supplementary motor area of the monkey's cerebral cortex: short- and long-term deficits after unilateral ablation and the effects of subsequent callosal section. J. Neurosci. 1984;4:918–929. doi: 10.1523/JNEUROSCI.04-04-00918.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkman J, Kuypers HG. Cerebral control of contralateral and ipsilateral arm, hand and finger movements in the split-brain rhesus monkey. Brain. 1973. pp. 653–674. [DOI] [PubMed]
- Kazennikov O. Temporal structure of a bimanual goal-directed movement sequence in monkeys. Eur. J. Neurosci. 1994;6:203–210. doi: 10.1111/j.1460-9568.1994.tb00262.x. [DOI] [PubMed] [Google Scholar]
- Kazennikov O. Neural activity of supplementary and primary motor areas in monkeys and its relation to bimanual and unimanual movement sequences. Neuroscience. 1999;89:661–674. doi: 10.1016/s0306-4522(98)00348-0. [DOI] [PubMed] [Google Scholar]
- Kermadi I, Liu Y, Tempini A, Rouiller EM. Effects of reversible inactivation of the supplementary motor area (SMA) on unimanual grasp and bimanual pull and grasp performance in monkeys. Somatosens. Mot. Res. 1997;14:268–280. doi: 10.1080/08990229770980. [DOI] [PubMed] [Google Scholar]
- Kermadi I, Liu Y, Tempini A, Calciati E, Rouiller EM. Neuronal activity in the primate supplementary motor area and the primary motor cortex in relation to spatio-temporal bimanual coordination. Somatosens. Mot. Res. 1998;15:287–308. doi: 10.1080/08990229870709. [DOI] [PubMed] [Google Scholar]
- Kermadi I, Liu Y, Rouiller EM. Do bimanual motor actions involve the dorsal premotor (PMd), cingulate (CMA) and posterior parietal (PPC) cortices? Comparison with primary and supplementary motor cortical areas. Somatosensory and Motor Research. 2000;17:255–271. doi: 10.1080/08990220050117619. [DOI] [PubMed] [Google Scholar]
- Wannier T, Liu J, Morel A, Jouffrais C, Rouiller EM. Neuronal activity in primate striatum and pallidum related to bimanual motor actions. NeuroReport. 2002;13:143–147. doi: 10.1097/00001756-200201210-00033. [DOI] [PubMed] [Google Scholar]
- Rouiller EM. Dexterity in adult monkeys following early lesion of the motor cortical hand area: the role of cortex adjacent to the lesion. Eur. J. Neurosci. 1998;10:729–740. doi: 10.1046/j.1460-9568.1998.00075.x. [DOI] [PubMed] [Google Scholar]
- Liu Y, Rouiller EM. Mechanisms of recovery of dexterity following unilateral lesion of the sensorimotor cortex in adult monkeys. Exp. Brain. Res. 1999;128:149–159. doi: 10.1007/s002210050830. [DOI] [PubMed] [Google Scholar]
- Freund P. Nogo-A-specific antibody treatment enhances sprouting and functional recovery after cervical lesion in adult primates. Nature. Med. 2006;12:790–792. doi: 10.1038/nm1436. [DOI] [PubMed] [Google Scholar]
- Freund P. Anti-Nogo-A antibody treatment promotes recovery of manual dexterity after unilateral cervical lesion in adult primates--re-examination and extension of behavioral data. Eur. J. Neurosci. 2009;29:983–996. doi: 10.1111/j.1460-9568.2009.06642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeser M. Effects of Unilateral Motor Cortex Lesion on Ipsilesional Hand's Reach and Grasp Performance in Monkeys: Relationship With Recovery in the Contralesional Hand. J. Neurophysiol. 2010;103:1603–1645. doi: 10.1152/jn.00459.2009. [DOI] [PubMed] [Google Scholar]
- Kaeser M. Autologous adult cortical cell transplantation enhances functional recovery following unilateral lesion of motor cortex in primates: a pilot study. Neurosurgery. 2011;68:1405–1417. doi: 10.1227/NEU.0b013e31820c02c0. [DOI] [PubMed] [Google Scholar]
- Bashir S. Short-term effects of unilateral lesion of the primary motor cortex (M1) on Ipsilesional hand dexterity in adult macaque monkeys. Brain Structure and Function. 2011. Forthcoming. [DOI] [PMC free article] [PubMed]
- Hamadjida A. Influence of anti-Nogo-A treatment on the reorganization of callosal connectivity of the premotor cortical areas following unilateral lesion of primary motor cortex (M1) in adult macaque monkeys. 2011. Forthcoming. [DOI] [PMC free article] [PubMed]
- Jenny AB, Inukai J. Principles of motor organization of the monkey cervical spinal cord. J. Neurosci. 1983;3:567–575. doi: 10.1523/JNEUROSCI.03-03-00567.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidlin E. Progressive plastic changes in the hand representation of the primary motor cortex parallel incomplete recovery from a unilateral section of the corticospinal tract at cervical level in monkeys. Brain Research. 2004;1017:172–183. doi: 10.1016/j.brainres.2004.05.036. [DOI] [PubMed] [Google Scholar]
- Schmidlin E. Reduction of the hand representation in the ipsilateral primary motor cortex following unilateral section of the corticospinal tract at cervical level in monkeys. BMC Neuroscience. 2005;6:56–56. doi: 10.1186/1471-2202-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott MJ. Refinement of the use of food and fluid control as motivational tools for macaques used in behavioural neuroscience research: report of a Working Group of the NC3Rs. J. Neurosci. Methods. 2010;193:167–188. doi: 10.1016/j.jneumeth.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Darling WG. Volumetric effects of motor cortex injury on recovery of dexterous movements. Exp. Neurol. 2009;220:90–108. doi: 10.1016/j.expneurol.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling WG. Minimal forced use without constraint stimulates spontaneous use of the impaired upper extremity following motor cortex injury. Exp. Brain. Res. 2010;202:529–542. doi: 10.1007/s00221-010-2157-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeal DW. Selective long-term reorganization of the corticospinal projection from the supplementary motor cortex following recovery from lateral motor cortex injury. J. Comp. Neurol. 2010;518:586–621. doi: 10.1002/cne.22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura Y. Time-dependent central compensatory mechanisms of finger dexterity after spinal cord injury. Science. 2007;318:1150–1155. doi: 10.1126/science.1147243. [DOI] [PubMed] [Google Scholar]
- Pizzimenti MA. Measurement of reaching kinematics and prehensile dexterity in nonhuman primates. J. Neurophysiol. 2007;98:1015–1029. doi: 10.1152/jn.00354.2007. [DOI] [PubMed] [Google Scholar]


