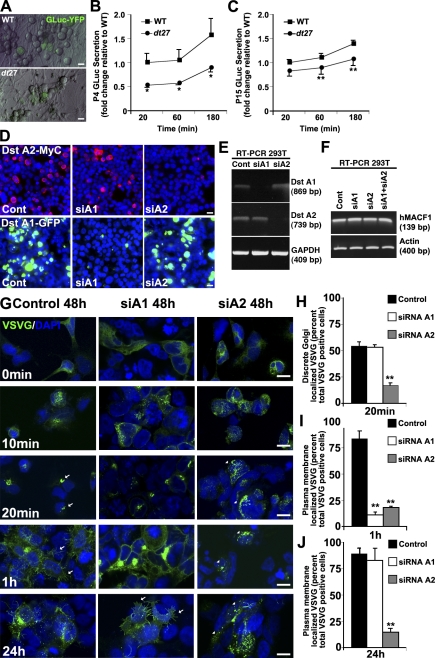
Figure 2.
Loss of dystonin impedes flux through the secretory pathway. Flux through the secretory pathway was assessed in primary sensory neurons. (A) GLuc was delivered to cells, and its secretion was monitored by measuring luciferase activity in the medium over time. (B and C) P4 (B) and P15 (C) dt27J neurons show a decrease in flux through the secretory pathway relative to WT sensory neurons. Data are expressed as fold change relative to WT 20 min (ANOVA posthoc Tukey; *, P < 0.05; n = 4–8). (D and E) Isoform-specific silencing of dystonin (Dst)-a1 and -a2 isoforms in 293T cells was confirmed on a protein (D) and mRNA (E) level. (F) Isoform-specific silencing of dystonin-a1 and -a2 isoforms or combined silencing in 293T cells has no impact on MACF1 mRNA levels. (G–J) Defective trafficking of VSVG from the ER to the Golgi after loss of dystonin-a2. Arrows depict Golgi-associated VSVG at 20 min and PM-associated VSVG at 1 h, whereas arrowheads depict aberrant VSVG localization (H). Although trafficking to the PM was retarded in both dystonin-a1 and dystonin-a2 knockdown cells at 1 h (G and I), only loss of dystonin-a2 showed a persisting defect at 24 h (G [arrows] and J). ANOVA posthoc Dunnett’s t test; **, P < 0.01; n = 6. Error bars show means ± SEM. Cont, control. Bars, 10 µm.