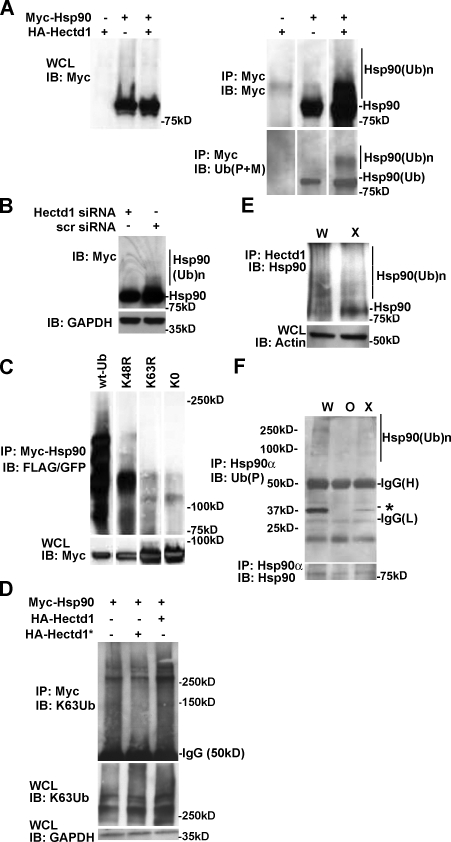
Figure 4.
Hectd1 is required for K63-linked Ubn of Hsp90. (A–D) HEK293T cells were transfected, and lysates were subjected to immunoprecipitation and Western blot analysis as indicated. (A) Ubiquitination of Myc-Hsp90 increases with expression of HA-Hectd1 (n = 2). (B) siRNA-mediated knockdown of endogenous Hectd1 reduces the accumulation of HMW-Hsp90α (n = 2). (C) Hsp90α ubiquitination utilizes K63 linkages. (D) Hectd1-dependent polyubiquitination of Hsp90 occurs primarily through K63 linkages. (E) HMW Hsp90 species are reduced in Hectd1 mutant heads. E12.5 Hectd1W (W) and Hectd1X (X) embryos were cultured in the presence of 10 µM MG132 for 3 h before lysis and immunoprecipitation of Hectd1. Immunoprecipitates were subjected to Western blot analyses to detect Hsp90 that coimmunoprecipitated with Hectd1. (F) Hsp90 ubiquitination is reduced in CM cultures from Hectd1O (O) and Hectd1X (X) mutants compared with Hectd1W (W). Hsp90 was immunoprecipitated from E12.5 CM primary cultures in highly denaturing ubiquitination buffer plus 5% SDS and subjected to Western blot analyses as indicated. The appearance of a 30-kD ubiquitinated protein (asterisk) is reduced in Hectd1 mutant cells. All data are representative of three independent experiments unless otherwise indicated. Abbreviations: W, Hectd1+/+; O, Hectd1opm/opm; X, Hectd1X/X; WCL, whole cell lysate; IB, immunoblot; IP, immunoprecipitation; (Ub)n, Ubn; wt-Ub, wild-type Ub; K48R, mutant Ub lysine 48 arginine; K63R, mutant Ub lysine 48 arginine; K0, lysineless Ub.