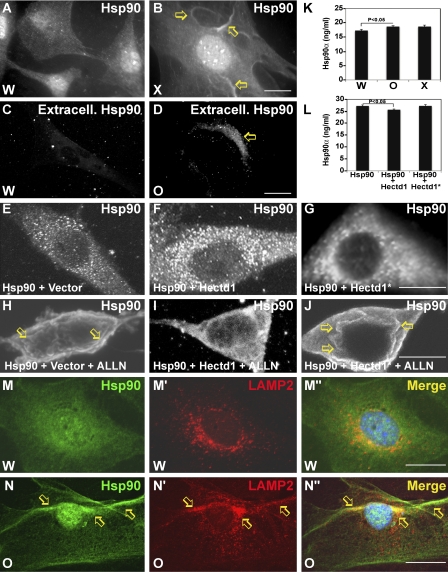
Figure 5.
Hectd1 affects Hsp90 localization and secretion. (A and B) Hectd1 Ub ligase activity is required for normal cytoplasmic distribution of Hsp90. (A–D) Hectd1W (A and C) and Hectd1X (B and D) CM cells were subjected to immunofluorescence analyses to detect total Hsp90 (A and B) or extracellular Hsp90 (C and D). Arrows indicate membrane concentrated Hsp90 (B) or extracellular Hsp90 (D). (E–J) Hectd1-dependent ubiquitination prevents ALLN-induced translocation of Hsp90 to the membrane. HEK293T cells transfected as indicated were treated (H–J) or not treated (E–G) with ALLN and immunostained to detect Myc-Hsp90. Arrows indicate membrane-concentrated Hsp90. (K) Mutation of Hectd1 results in increased secretion of Hsp90. ELISA was used to detect Hsp90α in conditioned media from Hectd1W, Hectd1O, and Hectd1x CM cultures. (L) Expression of HA-Hectd1 but not Ub ligase–deficient HA-Hectd1* reduced secretion of Hsp90 in HEK293T cells. Statistical significance was determined by paired two-tailed Student’s t tests. Error bars represent the mean ± SEM (SEM) of three independent experiments performed in triplicate. (M–N″) Hsp90 (M and N) colocalizes (M″ and N″) with LAMP2 (M′ and N′) in the membrane (arrows) of Hectd1O CM cells. All data are representative of three independent experiments unless otherwise indicated. W, Hectd1+/+; O, Hectd1opm/opm; X, Hectd1X/X. Bars, 10 µm.