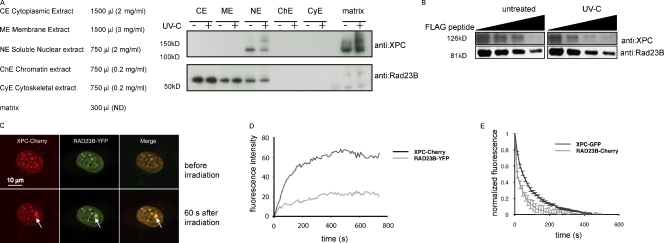
Figure 3.
The XPC–RAD23B complex dissociates upon UV-induced DNA damage. (A) Subcellular fractionation of human U2-OS with and without UV (60 min after 20 J/m2) treatment. The same volume was loaded on a gradient gel for each fraction. After Western blotting, membranes were stained with the indicated antibodies. (B) Immunoblot analysis for both XPC and RAD23B proteins of anti-FLAG–immunoprecipitated complexes from RAD23B-YFP-FLAG–expressing ES cells before and 60 min after UV-C (16 J/m2) treatment. Immunoprecipitated proteins were eluted with free FLAG peptide, and different fractions were collected, boiled, and separated on a gel. Upon elution with (free) Flag peptide, a lower amount of XPC coeluted when cells were treated with UV-C than from untreated cells. (C) Upon overexpression of XPC-Cherry (red channel), RAD23B-YFP does visibly accumulate at local damage (green channel), inflicted with a UV-C laser. Pictures of representative cells before (top row) and 60 s after (bottom row) UV-C are shown. Arrows indicate the spot of the local UV-C laser-induced DNA damage. (D) Relative accumulation of XPC-mCherry and RAD23B-YFP on local damage. Arbitrary fluorescence intensity units are plotted against the time (in seconds) after local damage infliction. (E) iFRAP, in which total fluorescence in the nuclei (except the fluorescence present at the local UV damage) is bleached and the subsequent loss in fluorescence of RAD23B-mCherry and XPC-GFP is monitored, which is a measure of the dissociation rate of these proteins from the local damage. RAD23B exhibits a quicker dissociation as compared with XPC, suggesting that RAD23B dissociates from XPC after binding of the complex to lesions. n = 10 cells. Error bars represent SEM.