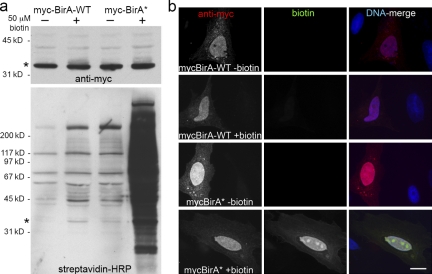
Figure 2.
BirA* promiscuously biotinylates endogenous proteins in mammalian cells. HeLa cells were analyzed 24 h after transient transfection with myc-BirA-WT or myc-BirA* (R118G). After transfection, cells were cultured either with or without supplemental biotin (50 µM). (a) By Western blot analysis similar levels of the exogenous BirA (asterisk) are detected in all samples with anti-myc. Biotinylated proteins, including both exogenous BirA (asterisk) and endogenous proteins, were detected with HRP-streptavidin. Enhanced protein biotinylation is observed in the myc-BirA* samples as compared with the WT isoform. This difference is dramatically enhanced by the presence of excess biotin. (b) By fluorescence microscopy the BirA is predominantly nuclear as observed with anti-myc (red). Biotinylated proteins were detected with fluorescently labeled streptavidin (green). Considerable biotinylation is only observed in cells expressing myc-BirA* and supplemented with excess biotin. The biotin signal predominantly colocalizes with myc-BirA*. DNA is labeled with Hoechst (blue). Bar, 4 µm.