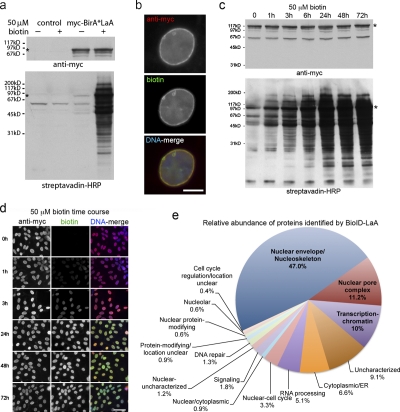
Figure 3.
Proximity-dependent promiscuous biotinylation by BioID-LaA. HEK293 cells inducibly expressing myc-BirA*LaA, or parental controls, were analyzed 24 h after induction with or without excess biotin. (a) By immunoblot analysis the LaA fusion protein (asterisk) is detected with anti-myc. In control cells levels of endogenously biotinylated proteins are unaffected by the supplemental biotin; however, the biotinylation of endogenous proteins by myc-BirA*LaA is dramatically enhanced in the presence of excess biotin. (b) The myc-BirA*LaA (red) is detected at the nuclear rim and to a lesser extent in the nucleoplasm. Biotinylated proteins (green) colocalize with the LaA fusion protein. DNA is labeled with Hoechst (blue). Bar, 5 µm. (c) To monitor the relative rate of biotinylation by BioID, myc-BirA*LaA HEK293 cells were provided with 50 µM biotin at different time points. The levels of myc-BirA*LaA (asterisk in panel a) are similar for all conditions; however, the extent of biotinylation increases with duration of biotin supplementation until it is saturated by 24 h. (d) Similar results were observed by fluorescence microscopy. Myc-BirA*LaA was detected with anti-myc (red) and biotinylated proteins with fluorescently labeled streptavidin (green). DNA was labeled with Hoechst (blue). Bar, 25 µm. (e) The identity of the proteins biotinylated by BioID-LaA was determined by mass spectrometry of proteins isolated with streptavidin-coupled magnetic beads. The relative abundance (percentage of total identified spectra adjusted for protein size, identified spectra per 103 amino acids) and classification of proteins uniquely biotinylated by myc-BirA*LaA is depicted in the chart. Conservatively estimated, 50% of the detected proteins predominantly reside at the nuclear lamina, INM, or nucleoplasmic face of the NPCs.