Table 1.
Reaction Optimization and Control Reactions.a
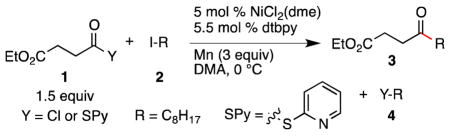 | ||||
|---|---|---|---|---|
| entry | Y | change in conditions | yield 3 (%)b | yield 4 (%) |
| 1 | Cl | None | 89 | 3 |
| 2 | Cl | No dtbpy | 8 | 7 |
| 3 | Cl | terpyridine in place of dtbpy | 27c | <1 |
| 4 | Cl | 1 equiv 1 instead of 1.5 equiv | 69 | 3 |
| 5 | Cl | 1.5 equiv 2, 1 equiv 1 | 56 | 2 |
| 6 | Cl | No nickel | 6d | 5 |
| 7 | Cl | No nickel, no dtbpy | 8d | 4 |
| 8 | Cl | No Mn | 0d | 25 |
| 9 | Cl | Zn in place of Mn | 41 | 1 |
| 10 | Spy | None | 37 | 43 |
| 11 | Spy | Zn in place of Mn | 53 | 25 |
| 12 | Spy | Pre-stir catalyst with Zn for 1 h | 71 | 10 |
| 13 | Spy | As in Entry 12, but 1 equiv 1 | 71 | 6 |
dtbpy = 4,4′-di-tert-butyl-2,2′-bipyridine. Terpyridine = 4,4′,4″-tri-tert-butyl-2,2′:6′,2″-terpyridine. Reactions were run on 0.5 mmol scale in 2 mL of N,N-dimethylacetamide (DMA). See Supporting Information for details.
Yield determined by GC analysis, uncorrected.
Alkyl dimer was the primary side-product (54% with respect to iodooctane). Alkyl dimer was not a significant byproduct in Entry 1.
>50% of R-I starting material remained.
