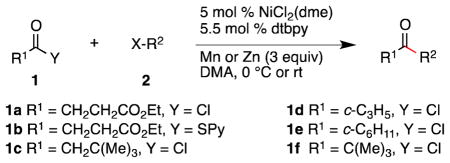
Table 2.
Scope of Ketone Synthesisa
 | ||||
|---|---|---|---|---|
| entry | 1 | 2 (R2-X) | product | yield (%)b |
| 1 | 1a | I-C8H17 (2a) |
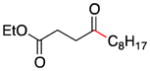 3 |
86 |
| 2c | 1b | 2a | 74 | |
| 3 | 1a |
2c |
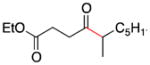 4 |
91 |
| 4d | 1a |
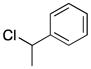 2d |
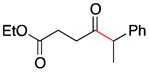 5 |
49e |
| 5 | 1c | 2c |
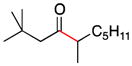 6 |
82 |
| 6 | 1d |
2e |
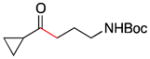 7 |
78 |
| 7 | 1e | 2c |
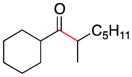 8 |
72 |
| 8f | 1e | 2d |
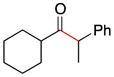 9 |
33 |
| 9 | 1f | 2e |
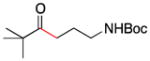 10 |
91 |
| 10 | 1f | 2c |
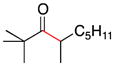 11 |
67 |
General conditions: 1.5 mmol of 1, 1.0 mmol of 2, 0.05 mmol NiCl2(dme), 0.055 mmol 4,4′-di-tert-butyl-2,2′-bipyridine (dtbpy), 3 mmol Mn, in 2 mL of DMA at 0 °C for 13–30 h.
Yield of isolated, purified product.
1 Equiv of thioester 1b was used in place of acid chloride 1a and Zn was used in place of Mn.
2 Equiv of 1a was used.
Product contained a small amount of diethyl succinate, yield of 5 determined by NMR.
Run at 30 °C instead of 0 °C.
