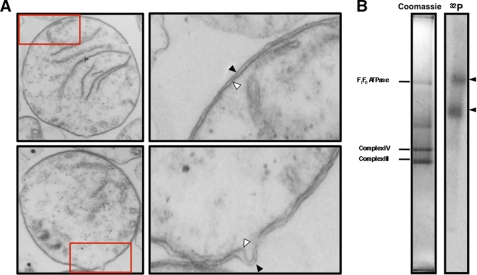
FIGURE 1.
Identification of tRNA binding complexes in extracts from T. brucei mitochondria. A, isolated mitochondria were characterized by transmission electron microscopy. Most mitochondria had intact outer (black arrowhead) and inner membranes (white arrowheads). A small fraction of mitochondria appear to have lost the outer membrane due to the hypotonic swelling and shearing protocol used in cell lysis (not shown). The areas designated by the red boxes in the lower magnification images were enlarged to better visualize the mitochondrial membranes (right panels). Total magnification: left panels, 27,272 ×; right panels, 89,089×. B, radiolabeled tRNALeu(CAA) binds two high molecular weight mitochondrial protein complexes (black arrowheads). Detergent extracts of T. brucei mitochondrial membrane were incubated with 32P-tRNALeu(CAA) and fractionated by blue native gel electrophoresis. The major mitochondrial membrane complexes visualized on stained blue native gel (left panel) were identified by LC-MS/MS as F1F0 ATP synthase (F1F0ATPase), cytochrome c oxidase (Complex IV), and cytochrome c reductase (Complex III) and were used as internal size standards.3 The tRNA binding complexes were detected by autoradiography (right panel).