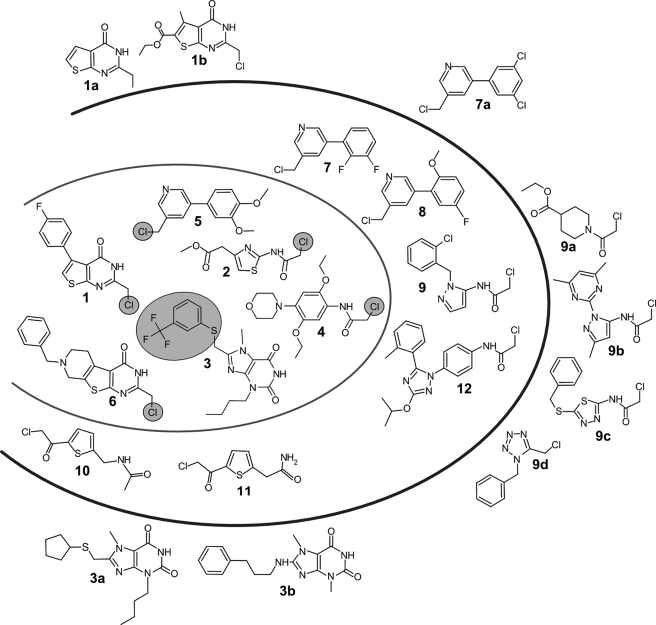
FIGURE 2.
Compounds active against the peroxidase cascade in the HTS and analogs identified by virtual screening. The 12 compounds within the outer oval revealed IC50-values of ≤31 μm. Compounds 1–6 (inner oval) displayed higher toxicity against T. brucei compared with HeLa cells (Table 1). The leaving group upon inactivation of Tpx is circled. The thienopyrimidine-4-ones 1, 1a, 1b, and 6, the purine 2,6-diones 3, 3a, and 3b, the chloromethyl-phenylpyridines 5, 7, 7a, and 8, as well as the chloroacetyl-thiophenes 10 and 11 each have a common core structure. Compounds 2, 4, 9, 9b, 9c, and 12 carry a 2-chloro-acetamido substituent. None of the analogues (marked by a–d) revealed pronounced inhibitory potency.