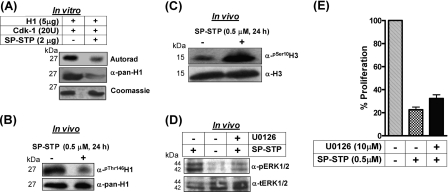
FIGURE 11.
A, autoradiogram and Coomassie staining showing the ability of SP-STP to dephosphorylate the CDK-1-phosphorylated histone H1 in vitro. The identity of the samples was ascertained by immunoblotting using anti-pan-H1 antibody. B, in vivo analysis of H1 phosphorylation in the nuclear extracts isolated from the control and SP-STP-treated pharyngeal cells using anti-phospho-Thr146-H1 (α-Thr(P)-146H1) antibody. Anti-H1 antibody-reactivity (α-pan-H1) was used as the loading control. C, anti-H3-Ser(P)10 antibody (α-Ser(P)-10-H3) reactivity showing histone H3 phosphorylation in the nuclear extracts of the SP-STP-treated and -untreated (control) pharyngeal cells. Anti-H3 antibody reactivity (Nu-H3) was used as the loading control. D, Western blot analysis depicting the change in the phospho-ERK1/2 (pERK1/2) profile in the untreated and SP-STP-treated pharyngeal cells alone or in the presence of the ERK1/2 inhibitor, U0126. The level of total ERK1/2 (tERK1/2) was taken as the loading control. E, MTT assay showing the SP-STP induced-proliferation inhibition of Detroit 562 cells in the presence and absence of ERK1/2 inhibitor U0126. The proliferation of cells treated with the inhibitor alone was taken as control.