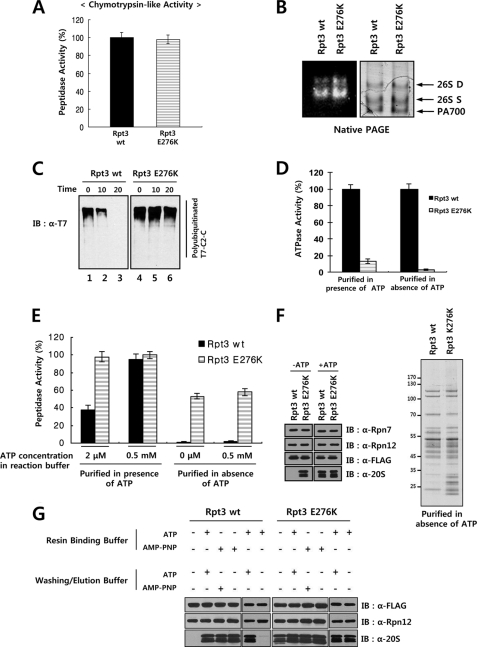
FIGURE 3.
Incorporation of a single Walker B mutant Rpt subunit abrogates the ATPase activity of 19 S RP. A, comparable chymotrysin-like activities of purified proteasome samples containing the wild-type or Walker B mutant Rpt3. One microgram each of the wild-type and Walker B mutant Rpt3 samples shown in Fig. 2C was assayed for chymotrypsin-like peptidase activity using the Suc-LLVY-AMC peptide as a fluorogenic substrate. B, native PAGE analysis and substrate overlay assay of proteasome samples containing wild-type or Walker B mutant Rpt3. Affinity-purified samples were subjected to native PAGE followed by Coomassie staining or by substrate overlay assay as described under “Experimental Procedures.” C, impaired degradation of a polyubiquitinated protein substrate by proteasome containing Walker B mutant Rpt3. Polyubiquitinated T7-cIAP2-C (C2-C) was prepared as described under “Experimental Procedures.” The polyubiquitinated substrate was incubated with indicated proteasome preparations and protein degradation was analyzed by Western blotting with anti-T7 antibody. D, impaired ATPase activity of proteasome containing Walker B mutant Rpt3. FLAG-Rpt3 samples purified in the presence or absence of ATP were assayed for ATPase activity as described under “Experimental Procedures.” E, chymotrysin-like activities of proteasome samples purified with or without ATP. Wild-type and Walker B mutant FLAG-Rpt3 samples purified in the presence or absence of ATP were assayed for chymotrypsin-like peptidase activity using Suc-LLVY-AMC as a fluorogenic substrate in buffers containing the indicated concentrations of ATP. Data are presented as the mean ± S.D. from three independent experiments. F, immunoblotting and SDS-PAGE analyses of proteasome samples purified with or without ATP. Wild-type and Walker B mutant FLAG-Rpt3 samples purified in the presence or absence of ATP were analyzed by immunoblotting with indicated antibodies. SDS-PAGE analysis of proteasome samples purified in the absence of ATP is shown in the right panel. G, increased stability of 26 S proteasome during purification by AMP-PNP. Wild-type and Walker B mutant FLAG-Rpt3 samples were purified using resin binding and washing/elution buffers with or without 2 mm ATP or AMP-PNP as indicated. Samples were analyzed by immunoblotting with indicated antibodies.