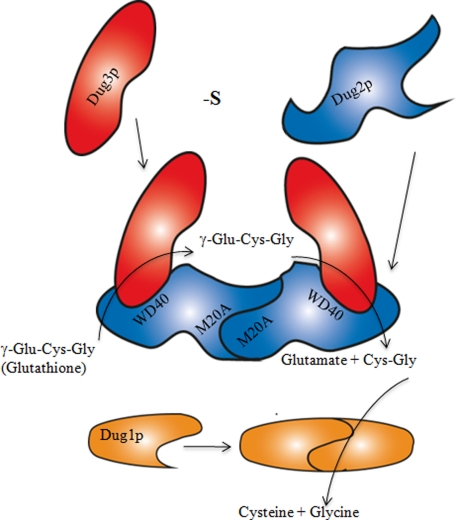
FIGURE 7.
Schematic diagram showing formation of (Dug2p-Dug3p)2 complex and role of Dug1p, Dug2p, and Dug3p proteins in glutathione degradation. Dug2p self-dimerizes to form a homodimer via its M20A peptidase domain at the N terminus. Both the subunits of the Dug2p homodimer interact with Dug3p by the WD40 domains at the C terminus of Dug2p. (Dug2p-Dug3p)2 complex can bind glutathione and cleave its γ-glutamyl linkage, thereby releasing Cys-Gly and glutamate. Cys-Gly released by the Dug2p-Dug3p complex is further acted upon by the Dug1p homodimer to form free cysteine and glycine. -S, 0 μm methionine.