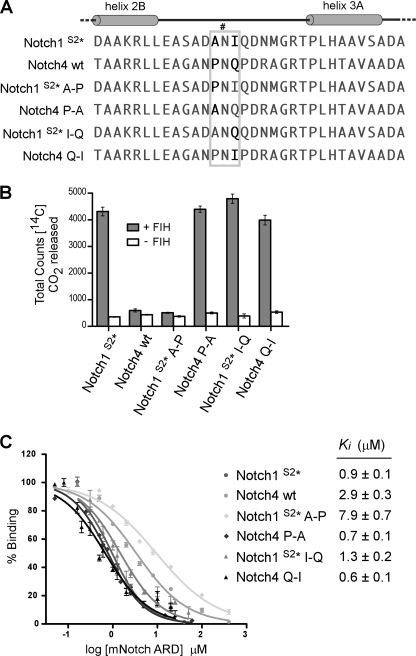
FIGURE 4.
Residues directly adjacent to the Asn in Notch influence hydroxylation and binding by FIH. A, shown is sequence alignment of hydroxylation Site 1, containing Asn-1945 (indicated by #), in mNotch1 and the corresponding region from the ARD of mNotch4, containing Asn-1656. Mutagenesis of the Notch1 and Notch4 ARDs was performed to generate Notch1S2* (N2012Q), Notch1S2* A-P (A1944P/N2012Q), Notch1S2* I-Q (I1946Q/N2012Q), Notch4 P-A (P1655A), and Notch4 Q-I (Q1657I). The gray box highlights the region in which the mutagenesis was performed. B, proteins described in A were tested as substrates for FIH in CO2 capture assays, with 50 μm concentrations of each ARD protein and a saturating amount (500 nm) of recombinant MBP-mouse FIH. Data are the mean of triplicate reactions ± S.D. and are representative of >3 independent experiments. C, affinity-purified Trx-6H-tagged Notch ARD proteins from B were assayed for their ability to compete with a fluorescently labeled Notch peptide in FP competition binding experiments. Ki values were determined using Graphpad PRISM software. Data are the average of three independent experiments ± S.E.