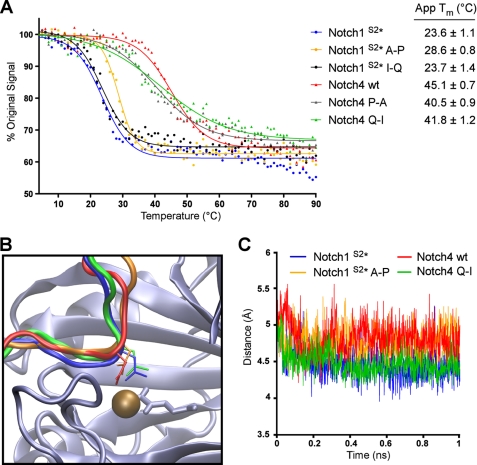
FIGURE 5.
ARD hydroxylation is influenced by thermodynamic stability and positioning of the target Asn. A, CD spectroscopy was employed to analyze the thermal denaturation of Trx-6H-tagged Notch wild type and mutant ARD proteins (described in Fig. 4A). The ellipticity at 220 nm (θ220) was monitored continuously as the temperature increased from 4 to 90 °C. Data are expressed as a percentage of the θ220 value at 4 °C, and apparent Tm values were determined using Graphpad PRISM software. A representative denaturation curve is shown for each protein, and apparent Tm values are the average of three independent experiments ± S.D. B, Notch peptide conformations during molecular dynamics simulation with FIH are shown. Schematic representation shows that peptides from the substrate proteins Notch1 wt (shown in blue) and Notch4 Q-I (green) exhibit a tighter turn around the target Asn residue, as compared with non-substrate peptides Notch1 A-P (orange) and Notch4 wt (red). C, this characteristic can be shown quantitatively throughout the 1-ns duration of molecular dynamics simulation via the measured distance between the Cα of the residue at the −1 position and the backbone N of the +1 residue.