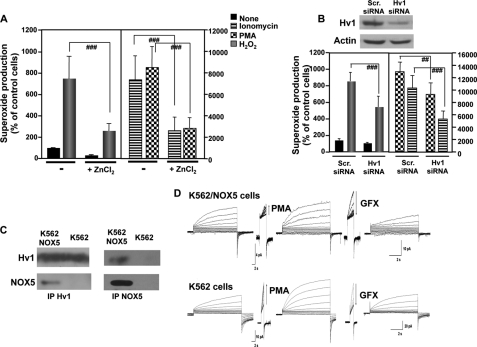
FIGURE 5.
Superoxide production by NOX5 requires HV1. A, superoxide production by K562/NOX5 cells preincubated for 5 min with or without 1 mm ZnCl2 and then stimulated with ionomycin (1 μm), PMA (1 μg/ml), or H2O2 (100 μm) was measured and expressed as described in the legend to Fig. 2. The data expressed are the mean ± S.E. of 6 independent experiments. Number signs indicate statistical significance versus stimulated cells in the absence of ZnCl2. ###, p < 0.001. B, K562/NOX5 cells were transiently transfected with scrambled (scr) siRNA or HV1-specific siRNA and tested for superoxide production stimulated by H2O2 (100 μm), ionomycin (1 μm), or PMA (1 μg/ml). Immunoblot analysis using HV1 antibody (above) was performed to ensure that the down-regulation of HV1 protein by siRNA was effective. Actin antibody was used to control for protein loading. The data expressed are the mean ± S.E. of 5 independent experiments. The number signs indicate statistical significance versus stimulated cells transfected with scrambled siRNA. ##, p < 0.01; ###, p < 0.001. C, immunoprecipitation of NOX5 or HV1 protein was performed using total protein lysates isolated from K562 cells or K562/NOX5 cells. The content of the immunoprecipitates was analyzed by immunoblot using HV1 and NOX5 antibodies. The results are representative of 3–5 experiments. D, families of proton currents were recorded during pulses in 10-mV increments up to +60 mV in K562 (lower tracings) and K562/NOX5 cells (upper tracings) before and then after stimulation with PMA (60 nm), and finally after addition of GFX (3 μm).