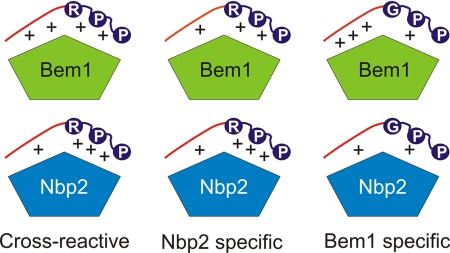
FIGURE 6.
Model of the BemSH3b and NbpSH3 domain peptide binding mechanisms. SH3 domains are shown as pentagons with the top two sides representing the distinct surfaces that interact with the core and extended regions of target peptides (Surface I and Surface II, respectively). The conserved Arg and Pro residues of the core motif are denoted with circles, and the extended regions of peptides are colored red. The plus signs symbolize the relative strengths of interactions between core and extended peptide regions and the domain binding surfaces. Interactions with four plus signs possess Kd values of less than 1 μm, three plus signs represent Kd values in the range of 20 μm, and two plus signs represents interactions with Kd values >90 μm. Cross-reactive sites, including Ste20 and Cla4(451–461), bind the NbpSH3 and BemSH3b domains with the same affinity despite different energetic contributions of the extended and core peptide regions toward binding. Nbp2-specific sites, including Skm1 and Bck1, bind the BemSH3b domain weakly due to suboptimal interactions with the extended region, but can still bind the NbpSH3 domain tightly because it is less dependent on these interactions. Bem1-specific sites, such as Boi1 and Cla4(15–25), interact weakly with the NbpSH3 domain due to suboptimal core motif interactions resulting from the lack of Arg−3. BemSH3b can still bind these sites with high affinity by compensating for weak interactions with the core motif through strong interactions with the extended region of these peptides.