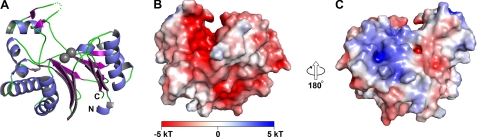
FIGURE 4.
Overall structural features of SUMO-BDP. A, ribbon representation of the structure. The α-helices are shown in blue, whereas the β-strands are drawn in purple. Regions without regular secondary structure are shown in green. A poorly ordered loop between residues 247 and 251 is shown as green dots. The N and C termini of the protein are labeled. The two bound metal ions are shown as gray spheres. B and C, surface and electrostatic surface features of the protein. The electrostatic potential of the protein (see “Experimental Procedures”) is drawn on a van der Waals surface representation. A legend to the color scheme is shown at the bottom of B. The view in B is the same as that in A. The arrows indicate that the view in C is 180° rotated from that in B.