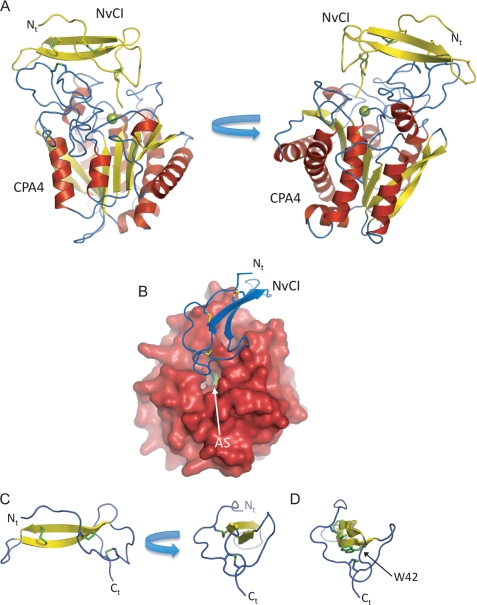
FIGURE 1.
Three-dimensional structure of NvCI in complex with human hCPA4. A, two views of the NvCI-hCPA4 complex shown in ribbon representation. The α-helix, β-strands, and coils of CPA4 are highlighted in red, yellow, and blue color, respectively. The NvCI structure is shown in yellow. The catalytic zinc atom in the metallocarboxypeptidase hCPA4 is shown in green. The three disulfide bridges formed in NvCI are shown in stick representation and visualized in green. The N terminus is labeled Nt. B, surface representation of hCPA4 in complex with NvCI shown in ribbon representation (blue). AS indicates the position of the active site groove of hCPA4. C, two views of the ribbon representation of NvCI. The β-strands and coils are colored in yellow and blue, respectively. The three disulfide bridges formed in NvCI are shown in stick representation (green). Ct, C terminus. D, same representation as in A but depicting Trp-42 in stick representation. All figures were prepared with PyMOL (32).