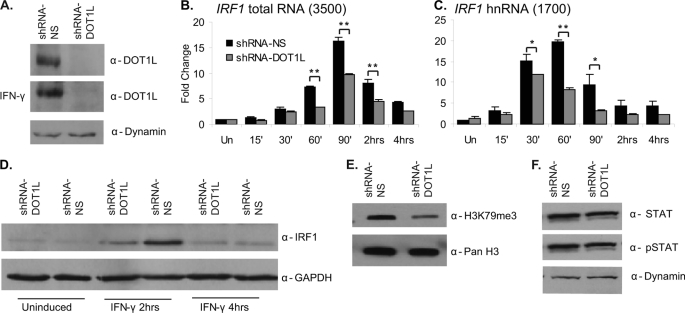
FIGURE 2.
RNAi-mediated depletion of DOT1L decreases IRF1 gene expression. A, DOT1L Western blot of whole cell extracts collected from 2fTGH cells stably expressing the pTRIPZ shRNAmir non-silencing (shRNA-NS) or shRNAmir-DOT1L (shRNA-DOT1L) vectors with or without IFN-γ. Dynamin served as a loading control. B and C, qRT-PCR to quantitate IRF1 mRNA and heteronuclear RNA (hnRNA) levels in the shRNAmir non-silencing and shRNAmir-DOT1L cell lines treated with IFN-γ for the times indicated or left untreated (Un). IRF1 was normalized to GAPDH, and expression is presented as -fold change relative to the uninduced, shRNAmir-NS condition. Error bars indicate S.E. (n = 3). Student's t test determined significance; **, p ≤ 0.01, *, p ≤ 0.05. D, IRF1 Western blot of whole cell extracts of shRNAmir non-silencing or shRNAmir-DOT1L cells treated with IFN-γ for the indicated times or left untreated. GAPDH served as a loading control. E, H3K79me3 Western blot of acid-extracted histones from the shRNAmir non-silencing and shRNAmir-DOT1L cell lines. Pan H3 served as a loading control. F, STAT1 and phospho-STAT1 Western blots of whole cell extracts collected from 2fTGH cells stably expressing the shRNAmir non-silencing or shRNAmir-DOT1L vectors. The ratio of phospho-STAT1 (pSTAT) to total STAT1 is the same in both cell lines. Western blot bands were quantified with ImageJ.