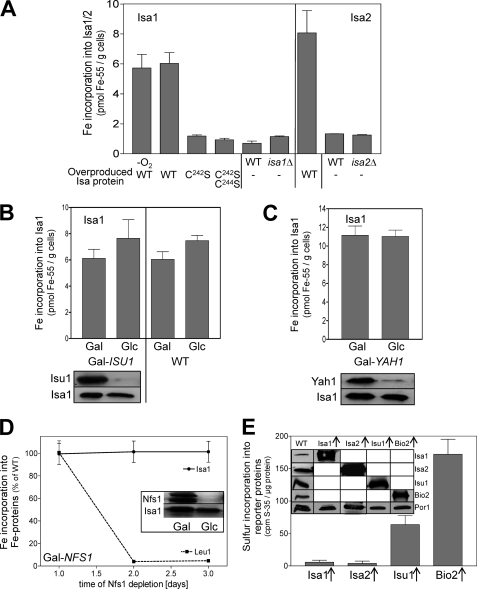
FIGURE 6.
Iron binding to Isa1 and Isa2 is independent of the sulfur supply from the mitochondrial ISC assembly machinery. A, 55Fe binding to Isa1 or Isa2 was determined by in vivo radiolabeling of wild-type cells overproducing either Isa1 (WT), the site-directed Isa1 mutant proteins C242S and C242S/C244S, or Isa2 (WT; right panel). Cell disruption and immunoprecipitation were performed under anaerobic (−O2) or aerobic (all other samples) conditions. isa1Δ, isa2Δ and WT cells containing the empty plasmid (−) served as controls. B and C, 55Fe binding to Isa1 was determined in Isa1-overproducing Gal-ISU1/Δisu2 or WT (B) and Gal-YAH1 cells (C) cultivated under inducing (Gal) or repressive conditions (Glc). Bottom panels of B and C show an immunostaining of the indicated proteins in cell extracts. D, Gal-NFS1 cells overproducing Isa1 were cultivated under repressive conditions in SD medium. At the indicated time points, 55Fe binding to Isa1 and Leu1 was determined in parallel by immunoprecipitation and scintillation counting. Inset, levels of the indicated proteins in cell extracts were assessed by immunostaining. E, detergent extracts of isolated mitochondria from wild-type cells overproducing (↑) Isa1, Isa2, Isu1, or Bio2 were radiolabeled with 10 μCi of [35S]cysteine for 2.5 h under anaerobic conditions. [35S]Sulfide binding was determined by immunoprecipitation and scintillation counting. Inset, protein levels in mitochondria from the indicated cells were assessed by immunostaining. Error bars, S.E. (n > 4).