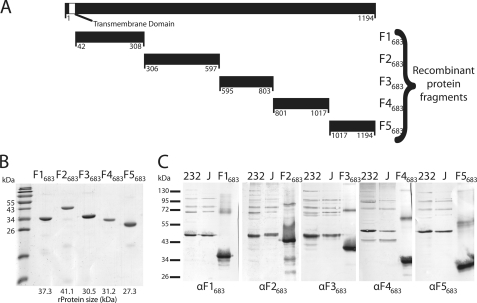
FIGURE 3.
Cloning of mhp683 and immunoblot analysis of its protein fragments. A, mhp683 (3582 bp encoding 1194 amino acids) was cloned from M. hyopneumoniae strain 232 as five fragments with sizes ranging from 178 to 292 amino acids. The hydrophobic putative transmembrane domain (41 amino acids) was removed from the N terminus of F1683. All in-frame TGA codons were mutated to TGG by using either mutated flanking primers or site-directed mutagenesis. B, Coomassie-stained SDS-PAGE of the five recombinant protein fragments in ascending order. Recombinant protein F3683 runs at a slightly higher molecular mass than expected on SDS-PAGE, and F1683 runs slightly lower. C, immunoblots of M. hyopneumoniae strain 232 and J whole cell lysates, and recombinant protein fragments (F1683–F5683) were probed with their respective antisera (αF1683–αF5683).