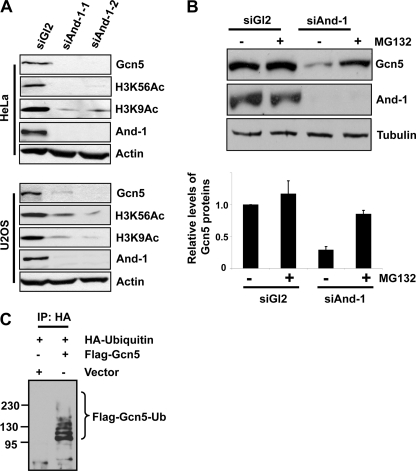
FIGURE 1.
Gcn5 is ubiquitinated in vivo and degraded by proteasome. A, depletion of And-1 results in the reduction of Gcn5 proteins and acetylation of histone H3K9 and H3K56 in both HeLa and U2OS cells. Cells treated with siRNA control (siGl2) or two independent siRNAs (siAnd-1-1 or siAnd-1-2) were harvested 48 h after siRNA transfection and subjected to immunoblot using anti-Gcn5, anti-H3K56Ac, anti-H3K9Ac, anti-And-1, or anti-actin antibody. B, treatment of And-1-depleted cells with the proteasome inhibitor MG132 stabilizes Gcn5 proteins. Upper, HeLa cells transfected with the indicated siRNAs were treated with (+) or without (-) MG132 (10 μm) and lysed. Lysates were subjected to immunoblot using anti-Gcn5, anti-And-1, or anti-tubulin antibody. Lower, quantification of Gcn5 protein levels. Means ± S.D. (error bars) were obtained from three independent experiments. The signals of Gcn5 and tubulin proteins were quantified by Scion imaging software, and the background signal was subtracted. The relative levels of Gcn5 proteins were normalized by control tubulin protein signals. The Gcn5 protein levels in siGl2-treated cells alone were taken as 100%. C, Gcn5 is ubiquitinated in vivo. U2OS cells were transfected with HA-ubiquitin and FLAG-Gcn5 or vector, and the ubiquitinated species were precipitated by HA antibodies and subjected to immunoblot using anti-FLAG antibody. IP, immunoprecipitation; Ub, ubiquitin.