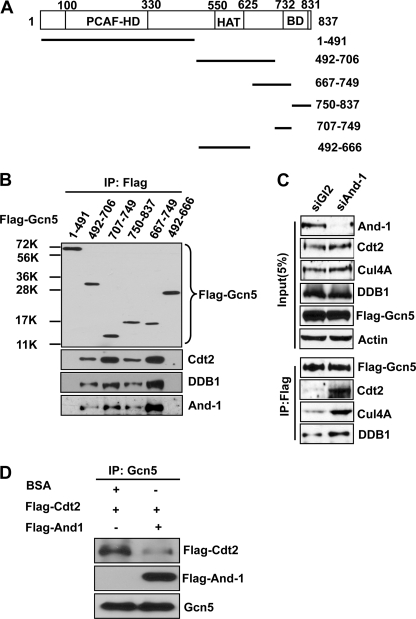
FIGURE 6.
And-1 competes with CRL4Cdt2 for the association with Gcn5. A, schematic of Gcn5 truncation mutants used for protein-protein interactions as in B. The P300/CBP-associated factor (PCAF) homology domain (PCAF-HD), histone acetyltransferase (HAT) domain, and bromo domain (BD) are indicated. B, And-1 and CRL4Cdt2 interact with Gcn5 at the same site within the C terminus. 293T cells transfected with the indicated plasmids were harvested and immunoprecipitated using anti-FLAG M2-agarose beads. FLAG-Gcn5 immunoprecipitates (IP) were subjected to immunoblot using anti-FLAG, anti-Cdt2, anti-DDB1, or anti-And-1 antibody. C, And-1 depletion increased the interaction between Gcn5 and CRL4Cdt2. U2OS cells transfected with the indicated siRNAs and FLAG-Gcn5 plasmid were treated with MG132 to stabilize Gcn5 before harvest. Cells were harvested 55 h after siRNA transfection for immunoprecipitation using anti-FLAG M2-agarose beads. FLAG-Gcn5 precipitates were subjected to immunoblot using anti-And-1, anti-Cdt2, anti-Cul4A, anti-actin, or anti-FLAG antibody. D, And-1 blocks the interaction between Cdt2 and Gcn5 in vitro. Immunopurified Gcn5 proteins on agarose beads were mixed with immunopurified FLAG-Cdt2 in the presence BSA or immunopurified FLAG-And-1 for 3 h at 4 °C. The beads were then washed three times using lysis buffer for immunoprecipitation. Gcn5 precipitates were subjected to immunoblot using anti-FLAG or anti-Gcn5 antibody.