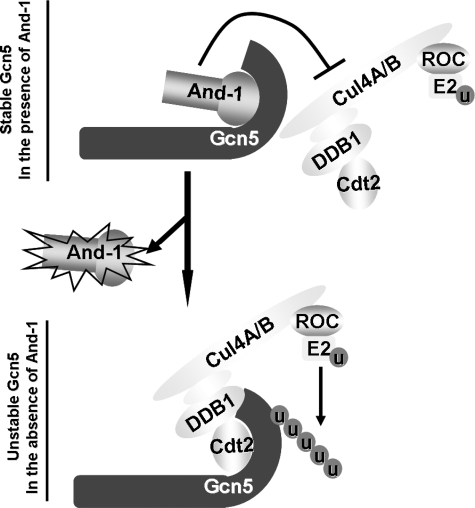
FIGURE 7.
A proposed model for the role of And-1 and CRL4Cdt2 in the regulation of Gcn5 protein turnover. Both And-1 and CRL4Cdt2 associate with Gcn5 at the same site within its C terminus. In the presence of And-1, the And-1 module associates with Gcn5 and stabilizes Gcn5 protein levels by preventing the interaction between Gcn5 and CRL4Cdt2 and thereby the ubiquitination of Gcn5. In the absence of And-1, CRL4Cdt2 interacts with the C terminus of Gcn5 and ubiquitinates Gcn5, resulting in the degradation of Gcn5. Note that the C-terminal domain of Cul4 associates with a small RING protein regulator of cullin (ROC), which recruits and activates E2 enzyme to transfer ubiquitin (u) to Gcn5.