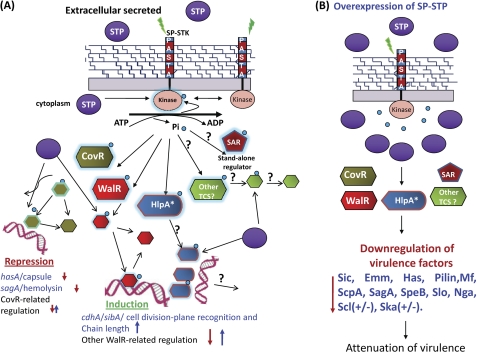
FIGURE 9.
Schematic representation of the regulatory role of the SP-STK/SP-STP couple in modulating GAS physiology and virulence. A, the external cues sensed by the wall-associated PASTA domain of SP-STK results in the activation of its cytoplasmically located kinase domain (STKK). The autophosphorylated (activated) SP-STK interfaces with certain well established TCSs (e.g. CovR and WalR) and possibly other stand-alone regulators (SAR). In that response, regulators of TCS get Thr-phosphorylated by SP-STK. The latter also recognizes other substrates, such as histone-like protein HlpA (7). The Thr-phosphorylated TCS regulators bind to specific promoters and regulate the transcription of several genes encoding for virulence factors such as capsule (Has), hemolysin (Sag), and CdhA. The schematic diagram summarizes the findings of the present study by showing that these function are cognately regulated by co-transcribing SP-STP, whose optimal balance in cytoplasm and its phosphatase activity at various levels may be required to allow or prevent the binding of regulators to its promoters and thus help maintain homeostasis within GAS (i.e. expression levels of various genes responsible for physiological/metabolic processes, growth, cell wall structure, cell division, and virulence). The absence of SP-STP thus allows uncontrolled SP-STK-mediated phosphorylation, which is manifested in the form of unique phenotypes. B, schematic diagram showing the implications of overexpression of SP-STP in GAS. Overexpression of SP-STP may dephosphorylate STK, and the Thr phosphorylation is abrogated even in the presence of appropriate external cues. This may result in down-regulation of virulence genes and attenuation and strain-specific differential expression of genes (M1SF370/M1T1). Normally secreted and overexpressed SP-STP may affect host cell signaling. + and ↑, up-regulation; − and ↓, down-regulation.