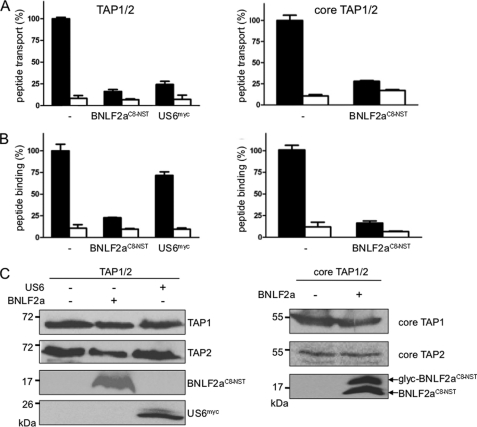
FIGURE 7.
BNLF2a targets the core TAP complex blocking peptide binding and transport. Full-length TAP (left panel) or core TAP (right panel) were expressed in Sf9 insect cells. BNLF2aC8-NST or US6myc were coexpressed as indicated. A, BNLF2a inhibits peptide transport by TAP. Equal amounts of membranes were incubated with RRYQNSTØL (1 μm, Ø, fluorescein-labeled cysteine) in the absence (apyrase, open bars) or presence of ATP (10 μm, filled bars) for 3 min at 32 °C. N-core glycosylated and thus translocated peptides were bound to ConA-beads and quantified by fluorescence (λex/em = 485/520 nm). Peptide transport by TAP was set to 100%. The means of at least three independent experiments are shown. Error bars indicate the S.D. B, BNLF2a inhibits peptide binding to TAP. Equal amounts of membranes were incubated with RRYØKSTEL (0.5 μm; Ø, fluorescein-labeled cysteine) for 15 min on ice (filled bars). A 100-fold excess of RRYQKSTEL was used to probe for unspecific binding (open bars). After washing on filter plates, the amount of membrane-associated peptide was quantified as described above. C, an equal amount of TAP in the membranes was confirmed by SDS-PAGE (10%) and immunoblotting with the corresponding antibodies. Glyc, glycosylated BNLF2a.