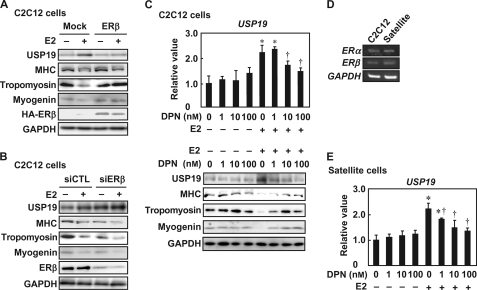
FIGURE 8.
Effects of ERβ knockdown and ERβ-selective agonist on E2-induced USP19 expression. A, C2C12 cells were transfected with pCAGGS-HA-ERβ for 24 h, followed by incubation in differentiation medium in the presence or absence of 10 nm E2 for 4 days. Cell lysates were subjected to Western blot analyses with anti-USP19, anti-MHC, anti-tropomyosin, anti-myogenin, anti-HA, and anti-GAPDH antibodies. B, C2C12 cells were transfected with ERβ siRNA (siERβ) and control siRNA (siCTL) for 24 h, followed by incubation in differentiation medium in the presence or absence of 10 nm E2 for an additional 4 days. Cell lysates were analyzed by Western blotting for USP19, MHC, tropomyosin, myogenin, ERβ, and GAPDH. C, C2C12 cells were differentiated in differentiation medium containing various concentrations of DPN in the presence or absence of 10 nm E2 for 8 days. Upper panel, qRT-PCR was performed, and USP19 mRNA level was normalized to GAPDH mRNA level. Lower panel, cell lysates were subjected to Western blot analyses for USP19, MHC, tropomyosin, myogenin, and GAPDH. D, satellite cells and C2C12 cells were cultured in differentiation medium in the presence of 10 nm E2 for 8 days. Semi-qRT-PCRs for ERα and ERβ mRNAs are shown. E, satellite cells were cultured in differentiation medium containing various concentrations of DPN in the presence or absence of 10 nm E2 for 8 days. USP19 and GAPDH mRNA levels were determined by qRT-PCR. Data were normalized to GAPDH. C and E, values are indicated as mean ± S.D. Statistically significant differences compared with the vehicle in the absence of E2 are indicated by *, p < 0.05. In the E2-treated cells, statistically significant differences compared with the vehicle are indicated by †, p < 0.05. In all experiments, data are representative of three independent experiments.