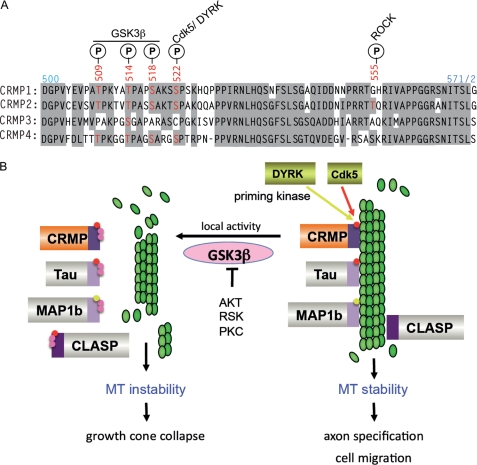
FIGURE 8.
How GSK3β phosphorylation is involved with microtubule destabilization. A, an alignment showing the conserved residues (shaded in gray) of the CMBD region of human CRMPs 1–4. Phosphorylation (P) sites (red) by kinases and GSK3β are indicated. B, multiple MT-binding proteins are co-regulated by phosphorylation. Pathways that lead to activation of priming kinases include Sema3A activation of CDK5 (66) (red) leading to growth cone collapse. The dual-tyrosine-regulated kinase (DYRK) family kinases (yellow) can activate CRMP4 as well as MAP1b (76). Local GSK3β activity leads to secondary phosphorylations (in pink) of MAPs, leading to their dissociation from microtubules and instability of the network. As shown, GSK3β can be antagonized by other protein kinases, which phosphorylate Ser-9 and could serve to terminate this pathway. CLASP, CLIP-associated protein; RSK, ribosomal S6 kinase.