Table 1.
Anti-HIV activities of 9a–9e in cellular assay and aqueous solubilitya
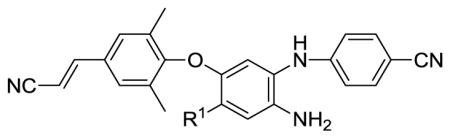
| ||||||
|---|---|---|---|---|---|---|
| R1 | EC50 (nM)b | CC50 (μM)c | SId | Aqueous Solubility (μg/mL)
|
||
| pH 7.4 | pH 2.0 | |||||
| 9a | COOCH3 | 2.74 ± 0.71 | >22.8 | >8465 | 1.30 | 1.75 |
| 9b | COOH | 230 ± 38 | 14.9 | 65 | 117 | 4.40 |
| 9c | CONH2 | 0.87 ± 0.28 | >23.6 | >27126 | 0.63 | 2.10 |
| 9d | CONHCH3 | 5.72 ± 0.94 | >9.2 | >1606 | 1.32 | 8.63 |
| 9e | CH2OH | 0.53 ± 0.13 | 16.3 | 30754 | 3.23 | 21.0 |
| 3e | NO2 | 0.38 ± 0.07 | >47.1 | >123947 | 0.29 | 0.29 |
| 2 (TMC278) | 0.52 ± 0.14 | 19.4 | 37305 | 0.24 | 156 | |
HIV-1 NL3-4 (wild-type) virus in TZM-bl cell lines.
Concentration of compound that causes 50% inhibition of viral replication and presented as mean ± standard deviation (SD) in at least triplicate.
Concentration of compound that causes cytotoxicity to 50% of cells.
Selectivity index (SI) is the ratio of CC50/EC50.
Compound and activity data were published previously12
