Figure 3.
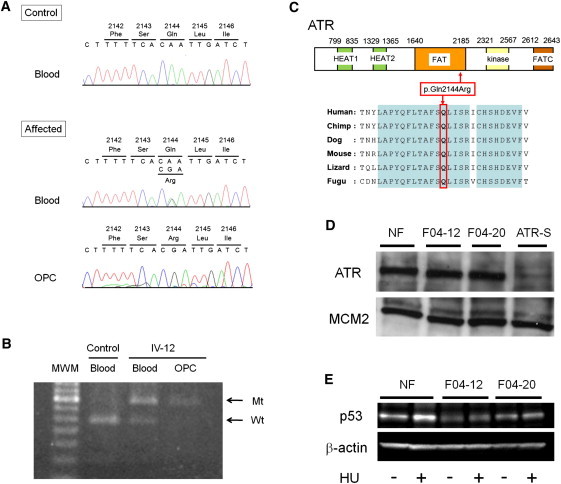
Identification of a Pathogenic Mutation in ATR
(A) Nucleotide sequencing of genomic DNA extracted from the blood of an affected individual reveals a heterozygous missense mutation, c.6431A>G (p.Gln2144Arg), in ATR (“affected blood”) but a homozygous or hemizygous mutation in an oropharyngeal-cancer lesion (“affected OPC”) from carrier individual IV-12.
(B) Analysis of the mutation c.6431A>G in ATR with restriction enzyme MfeI. PCR products from the wild-type (“Wt”) allele were digested, whereas those from the mutant (“Mt”) allele were not. Control-blood DNA shows only a digested band (“control blood”). DNA extracted from blood shows both an undigested band and a digested band (“IV-12 blood”) but only an undigested band from the oropharyngeal-cancer lesion (“IV-12 OPC”) in carrier individual IV-12.
(C) A schematic representation of ATR (NP_001175.2). The mutation occurs within the FAT domain of ATR. The substituted amino acid (p.Gln2144) is well conserved throughout various evolutionary lineages.
(D) Immunoblot of ATR in fibroblast extracts. “NF” indicates control fibroblasts from a healthy donor; “F04-12” and “F04-20” are fibroblasts from carriers IV-12 and IV-20, respectively, in Figure 1A; “ATR-S” denotes fibroblasts derived from an individual with ATR Seckel syndrome; MCM2 (minichromosome maintenance complex component 2) was used as a loading control. The antibodies sc-1887 (for ATR) and sc-9839 (for MCM2) were purchased from Santa Cruz (Santa Cruz, CA).
(E) Immunoblot of p53 in fibroblast extracts (of both carriers and controls) before and after the activation of ATR by hydroxyurea (“HU”) exposure for two hours; β-actin was used as a loading control. The antibodies #9282 (for p53) and #4967 (for β-actin) were purchased from Cell Signaling (Beverly, MA).
