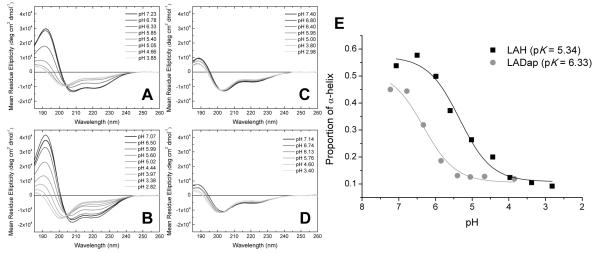
Figure 1.
pH dependent conformational changes in the four cationic amphipathic helical peptides. Dap containing LADap (A) and histidine containing LAH (B) undergo a helical to unordered transformation as the pH is lowered from pH 7. In contrast, lysine containing LAK (C) and ornithine containing LAO (D) retain an unordered conformation throughout the pH range tested. CDPro analysis of the pH dependent change in conformations for both LAH and LADap (E) indicates that the conformational change begins at a much higher pH for LADap than LAH.