Abstract
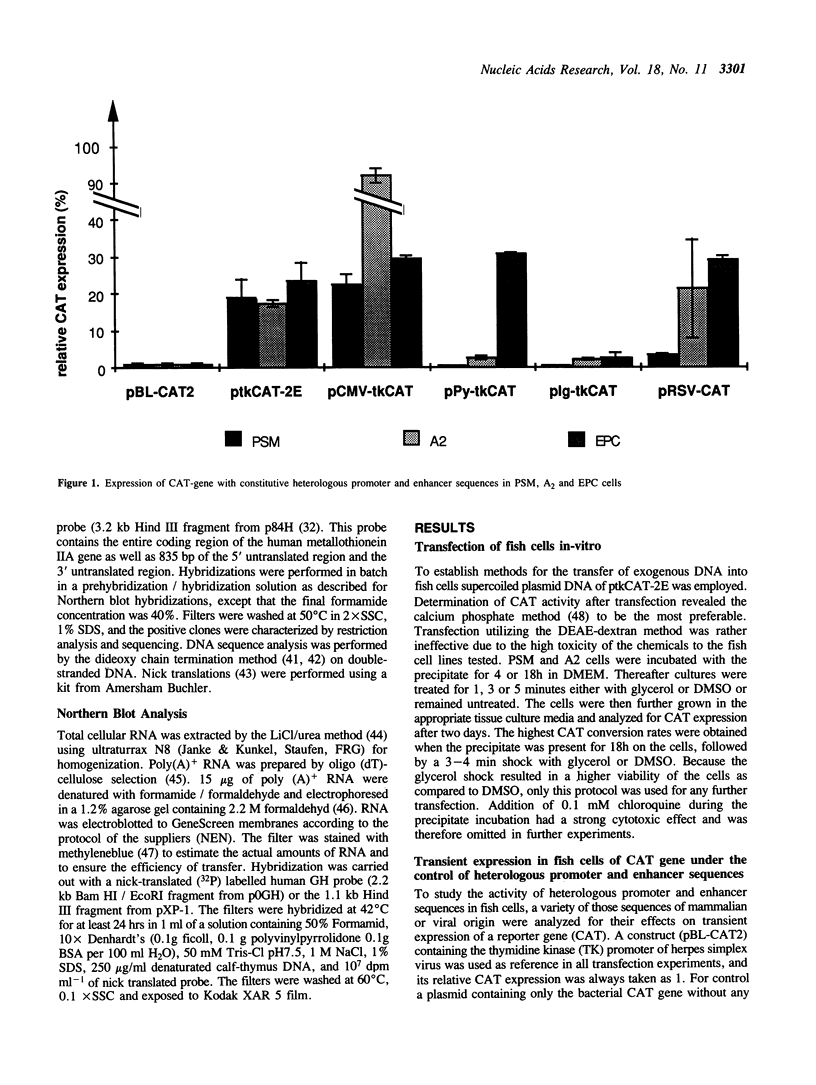
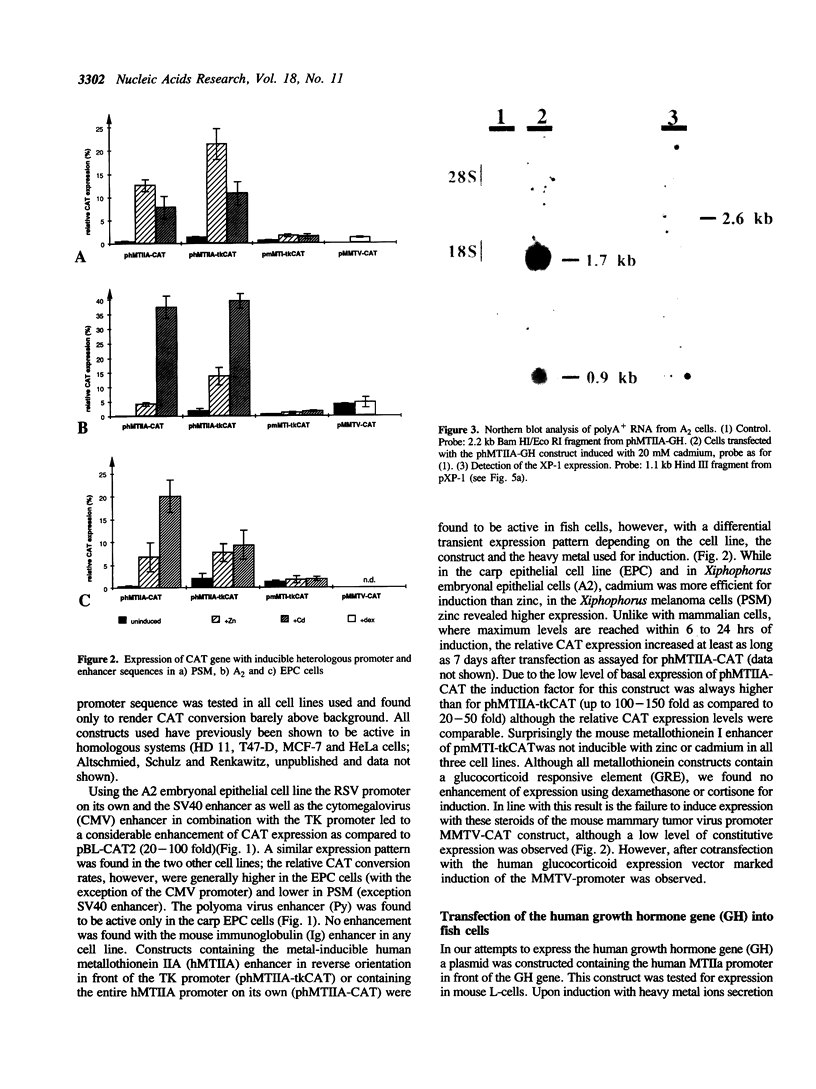
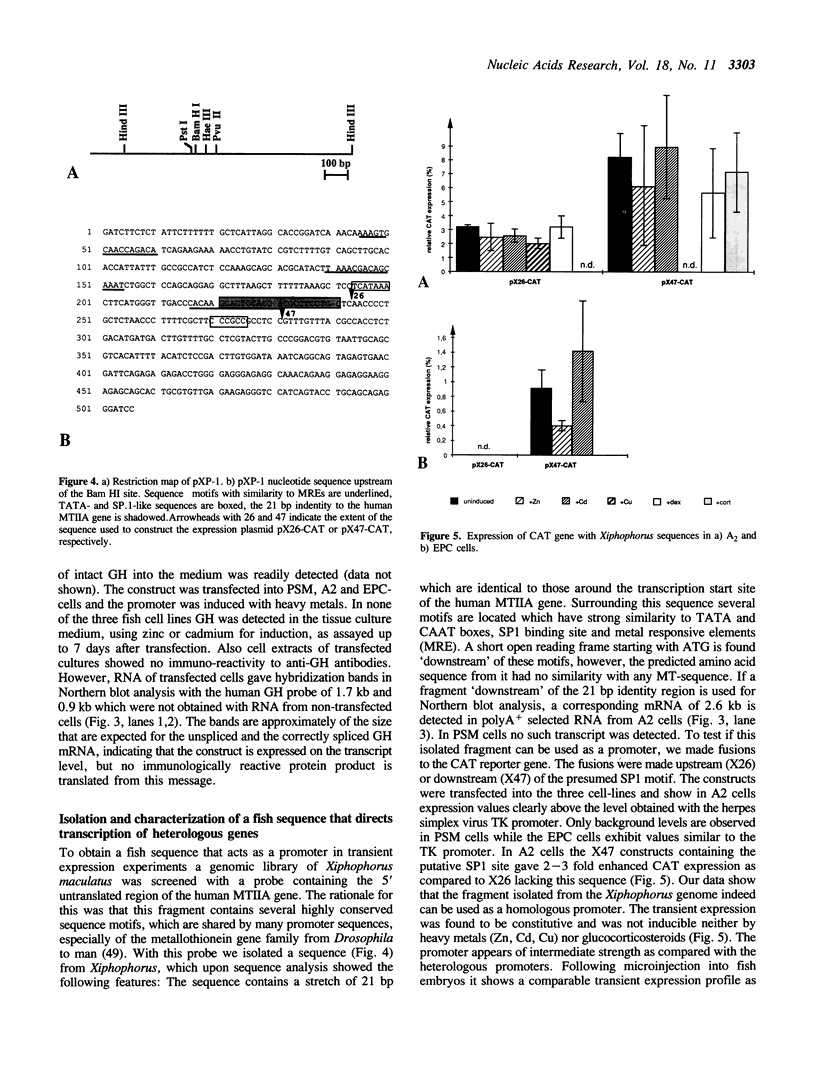
In order to construct fish specific expression vectors for studies on gene regulation in vitro and in vivo a variety of heterologous enhancers and promoters from mammals and from viruses of higher vertebrate cells were tested for expression of the bacterial chloramphenicol acetyl transferase reporter gene in three teleost fish cell lines. Several viral enhancers were found to be constitutively active at high levels. The human metallothionein promoter showed inducible expression in the presence of heavy metal ions. A fish sequence was isolated that can be used as a homologous constitutively active promoter for expression of foreign genes. Using the human growth hormone gene with an active promoter in fish cells for transient expression insufficient splicing and lack of translation were observed, pointing to limitations in the use of heterologous genes in gene transfer experiments. On the contrary, some heterologous promoters and enhancers functioned in fish cells as well as in their cell type of origin, indicating that corresponding transcription factors are sufficiently conserved between fish and human over a period of 900 million years of independent evolution.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agellon L. B., Chen T. T. Rainbow trout growth hormone: molecular cloning of cDNA and expression in Escherichia coli. DNA. 1986 Dec;5(6):463–471. doi: 10.1089/dna.1.1986.5.463. [DOI] [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Bonham K., Zafarullah M., Gedamu L. The rainbow trout metallothioneins: molecular cloning and characterization of two distinct cDNA sequences. DNA. 1987 Dec;6(6):519–528. doi: 10.1089/dna.1987.6.519. [DOI] [PubMed] [Google Scholar]
- Cato A. C., Miksicek R., Schütz G., Arnemann J., Beato M. The hormone regulatory element of mouse mammary tumour virus mediates progesterone induction. EMBO J. 1986 Sep;5(9):2237–2240. doi: 10.1002/j.1460-2075.1986.tb04490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang T., Chang W. C. Cloning and sequencing of a carp beta s-crystallin cDNA. Biochim Biophys Acta. 1987 Oct 9;910(1):89–92. doi: 10.1016/0167-4781(87)90098-4. [DOI] [PubMed] [Google Scholar]
- Eiken H. G., Njølstad P. R., Molven A., Fjose A. A zebrafish homeobox-containing gene with embryonic transcription. Biochem Biophys Res Commun. 1987 Dec 31;149(3):1165–1171. doi: 10.1016/0006-291x(87)90530-4. [DOI] [PubMed] [Google Scholar]
- Fjose A., Eiken H. G., Njølstad P. R., Molven A., Hordvik I. A zebrafish engrailed-like homeobox sequence expressed during embryogenesis. FEBS Lett. 1988 Apr 25;231(2):355–360. doi: 10.1016/0014-5793(88)80849-4. [DOI] [PubMed] [Google Scholar]
- Giguère V., Hollenberg S. M., Rosenfeld M. G., Evans R. M. Functional domains of the human glucocorticoid receptor. Cell. 1986 Aug 29;46(5):645–652. doi: 10.1016/0092-8674(86)90339-9. [DOI] [PubMed] [Google Scholar]
- González-Villaseñor L. I., Zhang P. J., Chen T. T., Powers D. A. Molecular cloning and sequencing of coho salmon growth hormone cDNA. Gene. 1988 May 30;65(2):239–246. doi: 10.1016/0378-1119(88)90460-x. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosschedl R., Baltimore D. Cell-type specificity of immunoglobulin gene expression is regulated by at least three DNA sequence elements. Cell. 1985 Jul;41(3):885–897. doi: 10.1016/s0092-8674(85)80069-6. [DOI] [PubMed] [Google Scholar]
- Guyomard R., Chourrout D., Leroux C., Houdebine L. M., Pourrain F. Integration and germ line transmission of foreign genes microinjected into fertilized trout eggs. Biochimie. 1989 Jul;71(7):857–863. doi: 10.1016/0300-9084(89)90050-3. [DOI] [PubMed] [Google Scholar]
- Haslinger A., Karin M. Upstream promoter element of the human metallothionein-IIA gene can act like an enhancer element. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8572–8576. doi: 10.1073/pnas.82.24.8572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeang K. T., Khoury G. The mechanistic role of enhancer elements in eukaryotic transcription. Bioessays. 1988 Apr;8(4):104–107. doi: 10.1002/bies.950080404. [DOI] [PubMed] [Google Scholar]
- Kadonaga J. T., Carner K. R., Masiarz F. R., Tjian R. Isolation of cDNA encoding transcription factor Sp1 and functional analysis of the DNA binding domain. Cell. 1987 Dec 24;51(6):1079–1090. doi: 10.1016/0092-8674(87)90594-0. [DOI] [PubMed] [Google Scholar]
- Karin M., Richards R. I. Human metallothionein genes--primary structure of the metallothionein-II gene and a related processed gene. Nature. 1982 Oct 28;299(5886):797–802. doi: 10.1038/299797a0. [DOI] [PubMed] [Google Scholar]
- Khandjian E. W. UV crosslinking of RNA to nylon membrane enhances hybridization signals. Mol Biol Rep. 1986;11(2):107–115. doi: 10.1007/BF00364822. [DOI] [PubMed] [Google Scholar]
- Kimmel C. B., Warga R. M. Cell lineage and developmental potential of cells in the zebrafish embryo. Trends Genet. 1988 Mar;4(3):68–74. doi: 10.1016/0168-9525(88)90043-1. [DOI] [PubMed] [Google Scholar]
- Kuhn C., Vielkind U., Anders F. Cell cultures derived from embryos and melanoma of poeciliid fish. In Vitro. 1979 Jul;15(7):537–544. doi: 10.1007/BF02618156. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Luckow B., Schütz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987 Jul 10;15(13):5490–5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miksicek R., Heber A., Schmid W., Danesch U., Posseckert G., Beato M., Schütz G. Glucocorticoid responsiveness of the transcriptional enhancer of Moloney murine sarcoma virus. Cell. 1986 Jul 18;46(2):283–290. doi: 10.1016/0092-8674(86)90745-2. [DOI] [PubMed] [Google Scholar]
- Nemoto N., Kodama K., Tazawa A., Masahito P., Ishikawa T. Extensive sequence homology of the goldfish ras gene to mammalian ras genes. Differentiation. 1986;32(1):17–23. doi: 10.1111/j.1432-0436.1986.tb00551.x. [DOI] [PubMed] [Google Scholar]
- Ozato K., Kondoh H., Inohara H., Iwamatsu T., Wakamatsu Y., Okada T. S. Production of transgenic fish: introduction and expression of chicken delta-crystallin gene in medaka embryos. Cell Differ. 1986 Dec;19(4):237–244. doi: 10.1016/0045-6039(86)90100-4. [DOI] [PubMed] [Google Scholar]
- Powers D. A. Fish as model systems. Science. 1989 Oct 20;246(4928):352–358. doi: 10.1126/science.2678474. [DOI] [PubMed] [Google Scholar]
- Price-Haughey J., Bonham K., Gedamu L. Heavy metal-induced gene expression in fish and fish cell lines. Environ Health Perspect. 1986 Mar;65:141–147. doi: 10.1289/ehp.8665141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raulf F., Mäueler W., Robertson S. M., Schartl M. Localization of cellular src mRNA during development and in the differentiated bipolar neurons of the adult neural retina in Xiphophorus. Oncogene Res. 1989;5(1):39–47. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott G. K., Hayes P. H., Fletcher G. L., Davies P. L. Wolffish antifreeze protein genes are primarily organized as tandem repeats that each contain two genes in inverted orientation. Mol Cell Biol. 1988 Sep;8(9):3670–3675. doi: 10.1128/mcb.8.9.3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine S., Mizukami T., Nishi T., Kuwana Y., Saito A., Sato M., Itoh S., Kawauchi H. Cloning and expression of cDNA for salmon growth hormone in Escherichia coli. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4306–4310. doi: 10.1073/pnas.82.13.4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selden R. F., Howie K. B., Rowe M. E., Goodman H. M., Moore D. D. Human growth hormone as a reporter gene in regulation studies employing transient gene expression. Mol Cell Biol. 1986 Sep;6(9):3173–3179. doi: 10.1128/mcb.6.9.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart G. W., McMurray J. V., Westerfield M. Replication, integration and stable germ-line transmission of foreign sequences injected into early zebrafish embryos. Development. 1988 Jun;103(2):403–412. doi: 10.1242/dev.103.2.403. [DOI] [PubMed] [Google Scholar]
- Stuart G. W., Searle P. F., Palmiter R. D. Identification of multiple metal regulatory elements in mouse metallothionein-I promoter by assaying synthetic sequences. 1985 Oct 31-Nov 6Nature. 317(6040):828–831. doi: 10.1038/317828a0. [DOI] [PubMed] [Google Scholar]
- Takeshita S., Aoki T., Fukumaki Y., Takagi Y. Cloning and sequence analysis of a cDNA for the alpha-globin mRNA of carp, Cyprinus carpio. Biochim Biophys Acta. 1984 Dec 14;783(3):265–271. doi: 10.1016/0167-4781(84)90037-x. [DOI] [PubMed] [Google Scholar]
- Van Beneden R. J., Watson D. K., Chen T. T., Lautenberger J. A., Papas T. S. Cellular myc (c-myc) in fish (rainbow trout): its relationship to other vertebrate myc genes and to the transforming genes of the MC29 family of viruses. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3698–3702. doi: 10.1073/pnas.83.11.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasylyk C., Imler J. L., Perez-Mutul J., Wasylyk B. The c-Ha-ras oncogene and a tumor promoter activate the polyoma virus enhancer. Cell. 1987 Feb 13;48(3):525–534. doi: 10.1016/0092-8674(87)90203-0. [DOI] [PubMed] [Google Scholar]
- Wasylyk C., Wasylyk B. The immunoglobulin heavy-chain B-lymphocyte enhancer efficiently stimulates transcription in non-lymphoid cells. EMBO J. 1986 Mar;5(3):553–560. doi: 10.1002/j.1460-2075.1986.tb04246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittbrodt J., Adam D., Malitschek B., Mäueler W., Raulf F., Telling A., Robertson S. M., Schartl M. Novel putative receptor tyrosine kinase encoded by the melanoma-inducing Tu locus in Xiphophorus. Nature. 1989 Oct 5;341(6241):415–421. doi: 10.1038/341415a0. [DOI] [PubMed] [Google Scholar]
- Zafarullah M., Bonham K., Gedamu L. Structure of the rainbow trout metallothionein B gene and characterization of its metal-responsive region. Mol Cell Biol. 1988 Oct;8(10):4469–4476. doi: 10.1128/mcb.8.10.4469. [DOI] [PMC free article] [PubMed] [Google Scholar]



