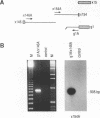
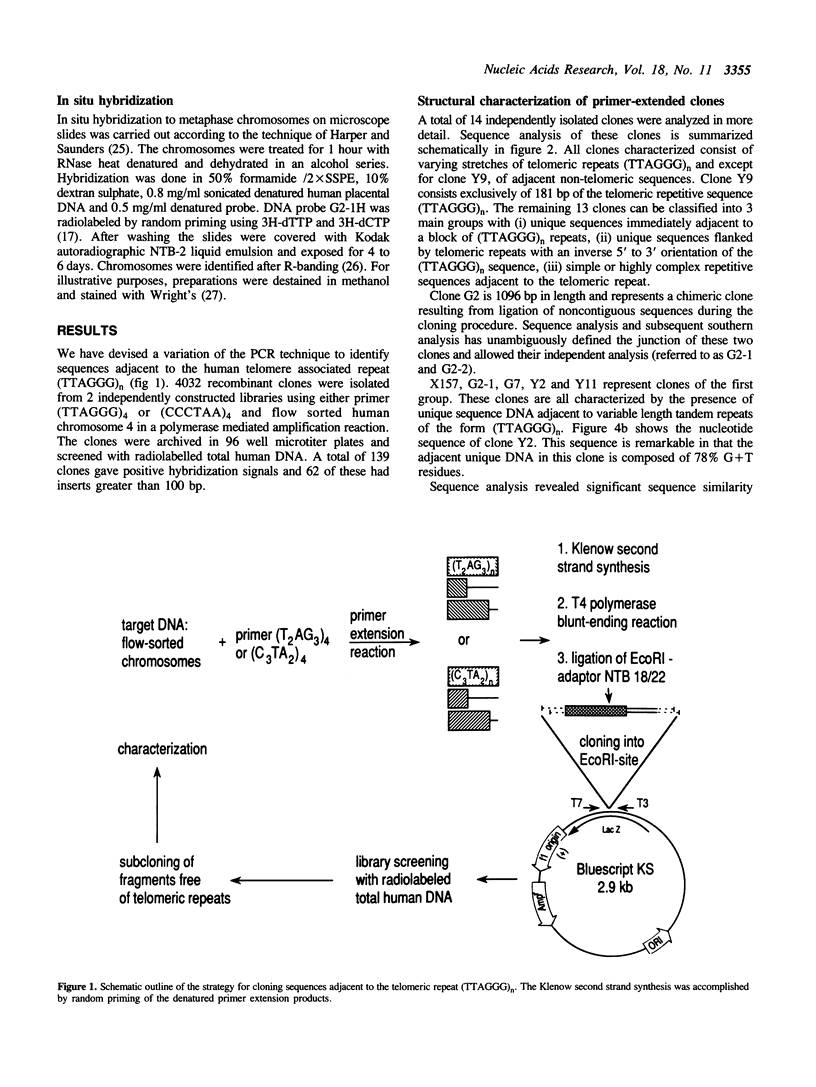
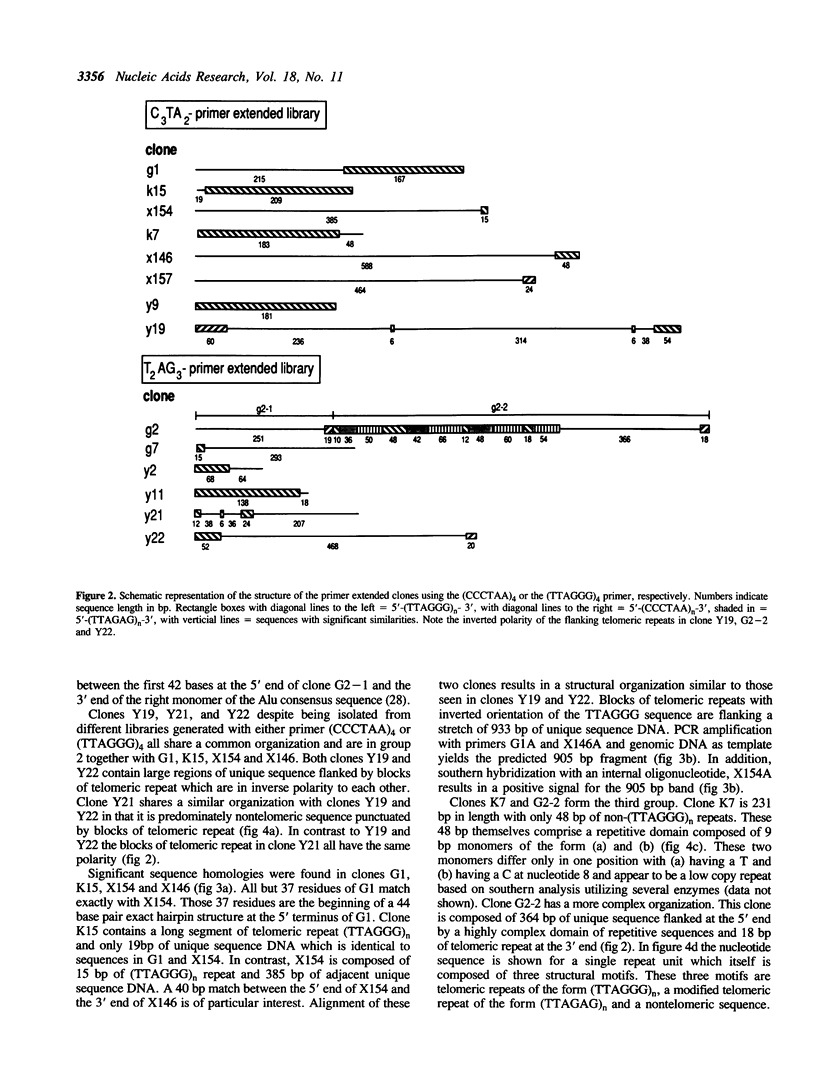
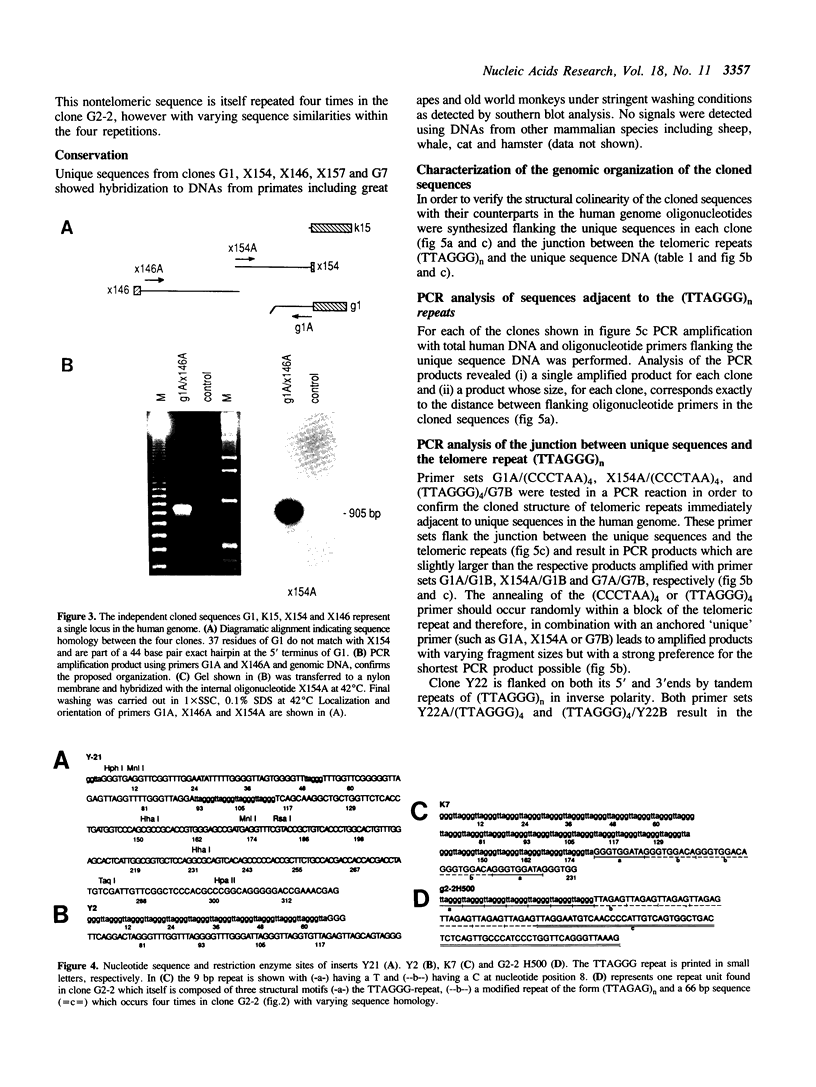
Abstract
We present a strategy for the cloning of DNA sequences adjacent to the tandemly repeated DNA sequence (TTAGGG)n. Sequence analysis of 14 independently isolated clones revealed the presence of non-repetitive sequences immediately adjacent to or flanked by blocks of the simple repeat (TTAGGG)n. In addition, we provide sequence information on two previously undescribed tandemly repeated sequences, including a 9 bp repeat and a modification of the (TTAGGG)n repeat. Using different mapping approaches six sub-clones, free of the TTAGGG repeat, were assigned to a single human chromosome. Moreover, in situ hybridization mapped one of these subclones, G2 - 1H, definitively to the telomeric band on chromosome 4q. However, Bal 31 insensitivity suggests a location in a more subterminal region. All the (TTAGGG)n-adjacent unique sequences tested are highly conserved among primates but are not present in other mammalian species. Identification and mapping of TTAGGG-adjacent sequences will provide a refined insight into the genomic organization of the (TTAGGG)n repeat. The isolation of chromosome specific TTAGGG-adjacent sequences from subtelomeric regions of all human chromosomes will serve as important end points for the genetic maps and will be useful for the molecular characterization of chromosomal rearrangements involving telomeres.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allshire R. C., Dempster M., Hastie N. D. Human telomeres contain at least three types of G-rich repeat distributed non-randomly. Nucleic Acids Res. 1989 Jun 26;17(12):4611–4627. doi: 10.1093/nar/17.12.4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn E. H., Budarf M. L., Challoner P. B., Cherry J. M., Howard E. A., Katzen A. L., Pan W. C., Ryan T. DNA termini in ciliate macronuclei. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):1195–1207. doi: 10.1101/sqb.1983.047.01.135. [DOI] [PubMed] [Google Scholar]
- Blackburn E. H., Challoner P. B. Identification of a telomeric DNA sequence in Trypanosoma brucei. Cell. 1984 Feb;36(2):447–457. doi: 10.1016/0092-8674(84)90238-1. [DOI] [PubMed] [Google Scholar]
- Blackburn E. H. Telomeres: do the ends justify the means? Cell. 1984 May;37(1):7–8. doi: 10.1016/0092-8674(84)90295-2. [DOI] [PubMed] [Google Scholar]
- Blackburn E. H. The molecular structure of centromeres and telomeres. Annu Rev Biochem. 1984;53:163–194. doi: 10.1146/annurev.bi.53.070184.001115. [DOI] [PubMed] [Google Scholar]
- Brown W. R. Molecular cloning of human telomeres in yeast. Nature. 1989 Apr 27;338(6218):774–776. doi: 10.1038/338774a0. [DOI] [PubMed] [Google Scholar]
- Cheng J. F., Smith C. L., Cantor C. R. Isolation and characterization of a human telomere. Nucleic Acids Res. 1989 Aug 11;17(15):6109–6127. doi: 10.1093/nar/17.15.6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke H. J., Brown W. R., Rappold G. A. Hypervariable telomeric sequences from the human sex chromosomes are pseudoautosomal. Nature. 1985 Oct 24;317(6039):687–692. doi: 10.1038/317687a0. [DOI] [PubMed] [Google Scholar]
- Cooke H. J., Smith B. A. Variability at the telomeres of the human X/Y pseudoautosomal region. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):213–219. doi: 10.1101/sqb.1986.051.01.026. [DOI] [PubMed] [Google Scholar]
- Cross S. H., Allshire R. C., McKay S. J., McGill N. I., Cooke H. J. Cloning of human telomeres by complementation in yeast. Nature. 1989 Apr 27;338(6218):771–774. doi: 10.1038/338771a0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Gray J. W., Dean P. N., Fuscoe J. C., Peters D. C., Trask B. J., van den Engh G. J., Van Dilla M. A. High-speed chromosome sorting. Science. 1987 Oct 16;238(4825):323–329. doi: 10.1126/science.2443974. [DOI] [PubMed] [Google Scholar]
- Greider C. W., Blackburn E. H. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature. 1989 Jan 26;337(6205):331–337. doi: 10.1038/337331a0. [DOI] [PubMed] [Google Scholar]
- Harper M. E., Saunders G. F. Localization of single copy DNA sequences of G-banded human chromosomes by in situ hybridization. Chromosoma. 1981;83(3):431–439. doi: 10.1007/BF00327364. [DOI] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Kariya Y., Kato K., Hayashizaki Y., Himeno S., Tarui S., Matsubara K. Revision of consensus sequence of human Alu repeats--a review. Gene. 1987;53(1):1–10. doi: 10.1016/0378-1119(87)90087-4. [DOI] [PubMed] [Google Scholar]
- McClintock B. The Stability of Broken Ends of Chromosomes in Zea Mays. Genetics. 1941 Mar;26(2):234–282. doi: 10.1093/genetics/26.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyne J., Ratliff R. L., Moyzis R. K. Conservation of the human telomere sequence (TTAGGG)n among vertebrates. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7049–7053. doi: 10.1073/pnas.86.18.7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin G. B. The human telomere terminal transferase enzyme is a ribonucleoprotein that synthesizes TTAGGG repeats. Cell. 1989 Nov 3;59(3):521–529. doi: 10.1016/0092-8674(89)90035-4. [DOI] [PubMed] [Google Scholar]
- Moyzis R. K., Buckingham J. M., Cram L. S., Dani M., Deaven L. L., Jones M. D., Meyne J., Ratliff R. L., Wu J. R. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6622–6626. doi: 10.1073/pnas.85.18.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters D., Branscomb E., Dean P., Merrill T., Pinkel D., Van Dilla M., Gray J. W. The LLNL high-speed sorter: design features, operational characteristics, and biological utility. Cytometry. 1985 Jul;6(4):290–301. doi: 10.1002/cyto.990060404. [DOI] [PubMed] [Google Scholar]
- Petit C., Levilliers J., Weissenbach J. Physical mapping of the human pseudo-autosomal region; comparison with genetic linkage map. EMBO J. 1988 Aug;7(8):2369–2376. doi: 10.1002/j.1460-2075.1988.tb03081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluta A. F., Zakian V. A. Recombination occurs during telomere formation in yeast. Nature. 1989 Feb 2;337(6206):429–433. doi: 10.1038/337429a0. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer D. Simultaneous fluorescent staining of R bands and specific heterochromatic regions (DA-DAPI bands) in human chromosomes. Cytogenet Cell Genet. 1980;27(2-3):190–193. doi: 10.1159/000131482. [DOI] [PubMed] [Google Scholar]
- Sen D., Gilbert W. Formation of parallel four-stranded complexes by guanine-rich motifs in DNA and its implications for meiosis. Nature. 1988 Jul 28;334(6180):364–366. doi: 10.1038/334364a0. [DOI] [PubMed] [Google Scholar]
- Smith B., Skarecky D., Bengtsson U., Magenis R. E., Carpenter N., Wasmuth J. J. Isolation of DNA markers in the direction of the Huntington disease gene from the G8 locus. Am J Hum Genet. 1988 Feb;42(2):335–344. [PMC free article] [PubMed] [Google Scholar]
- Wyman A. R., Wolfe L. B., Botstein D. Propagation of some human DNA sequences in bacteriophage lambda vectors requires mutant Escherichia coli hosts. Proc Natl Acad Sci U S A. 1985 May;82(9):2880–2884. doi: 10.1073/pnas.82.9.2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunis J. J., Chandler M. E. High-resolution chromosome analysis in clinical medicine. Prog Clin Pathol. 1978;7:267–288. [PubMed] [Google Scholar]