Table 2.
 | |||||
|---|---|---|---|---|---|
| Entry | R | Ar | Ar1 | d.r. | Yield [%] |
| 1 | 4-ClPh | Ph | Ph | >20 : 1 | 94 (4) |
| 2 | 4-ClPh | 4-BrPh | Ph | >20 : 1 | 87 (5) |
| 3 | 4-ClPh | 4-MePh | Ph | >20 : 1 | 61 (6) |
| 4 | 4-ClPh | 4-ClPh | Ph | >20 : 1 | 76 (7) |
| 5 | 4-ClPh | 2-Napth | Ph | >20 : 1 | 74 (8) |
| 6 | 4-ClPh | 2-ClPh | Ph | >20 : 1 | 75 (9) |
| 7 | 4-ClPh | 4-MeOPh | Ph | >20 : 1 | 71 (10)c |
| 8 | Ph | 2-ClPh | Ph | >20 : 1 | 81 (11)c |
| 9 | Ph | 4-MeOPh | Ph | >20 : 1 | 63 (12)c |
| 10 | Ph | 4-MePh | Ph | >20 : 1 | 70 (13)c |
| 11 | Ph | 2-Napth | Ph | >20 : 1 | 77 (14)c |
| 12 | 4-MePh | 4-ClPh | Ph | >20 : 1 | 75 (15)c |
| 13 | PhCH2 | Ph | Ph | >20 : 1 | 86 (16)c |
| 14 | 4-ClPh | Ph | 4-MePh | >20 : 1 | 72 (17)d |
| 15 | 4-ClPh | Ph | 4-BrPh | >20 : 1 | 60 (18)d |
| 16 | Ph | Ph | 4-MePh | >20 : 1 | 77 (19)d |
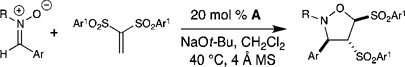
General conditions: vinyl sulfone (1 equiv), nitrone (1.05 equiv), azolium salt A (20 mol%), NaOt-Bu (20 mol%), CH2Cl2 (0.10 M).
Yield of isolated products.
Vinyl sulfone (2 equiv), nitrone (1 equiv).
1,2-dichloroethane (0.10 M), 40 °C, no 4 Å MS.
