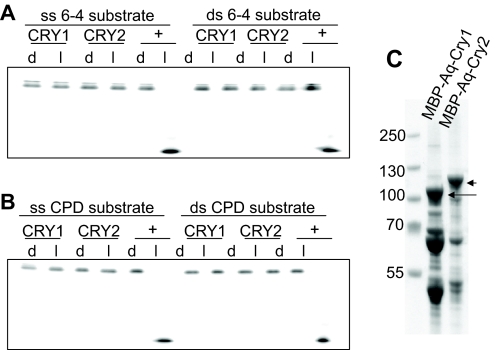
Fig. 2.
DNA repair ability of A. queenslandica cryptochromes. A 48-nt-long radiolabeled oligomer with photoproducts in the TTAA sequence (MseI recognition site) in the center, in single-stranded (ss) or double-stranded (ds) form, was mixed with the appropriate enzyme and exposed to photoreactivating light (l) or kept in the dark (d). The level of repair was determined by the susceptibility of the DNA to cleavage by MseI endonuclease. Repaired products were annealed with the complementary strand (1:100 fold) before digestion with MseI. The reaction products were separated on 8% polyacrylamide gels. (A) Single- and double-stranded oligos containing CPD dimers were incubated in 100 nmol l–1 of Aq-Cry1, Aq-Cry2, and positive controls Arabidopsis DASH photolyase (ss substrate) and Caulobacter cresenteus photolyase (ds substrate) for 90 min in the dark or 2 mW cm–2 black light. Oligos with repaired photoproducts became sensitive to MseI digestion, resulting in a shorter oligo. In these conditions, Aq-Cry1 and Aq-Cry2 did not repair photoproducts, indicating that they do not have any photolyase activity in vitro. (B) Single- and double-stranded oligos containing [6-4] photoproducts were incubated in 100 nmol l–1 of Aq-Cry1, Aq-Cry2, or positive control Xenopus 6-4 photolyase (ss and ds substrate) for 90 min in the dark or 2 mW cm–2 black light. Oligos with repaired photoproducts became sensitive to MseI digestion, resulting in a shorter oligonucleotide. In these conditions, Aq-Cry1 and Aq-Cry2 did not repair photoproducts, indicating that they do not have any photolyase activity in vitro. (C) Analysis of purified protein by 4–12% gradient SDS-PAGE. The numbers on the left indicate size markers. Lane 1, purified MBP-AqCRY1; lane 2, purified MBP-AqCRY2 through amylose resin. The positions of full-length CRYs are indicated by arrows. Extensive washing of the amylose resin by high-salt buffer causes considerable loss of the flavin co-factor. Therefore, the proteins bound to amylose resin were washed with two column volumes of buffer with moderate ionic strength before elution with maltose, and the preparation contains contaminating proteins at a considerable level. However, because the recombinant proteins were expressed in the photolyase-deficient E. coli strain UNC523 (phr::kan uvrA::Tn10), the contaminating proteins do not interfere with subsequent in vitro photoreactivation assays.