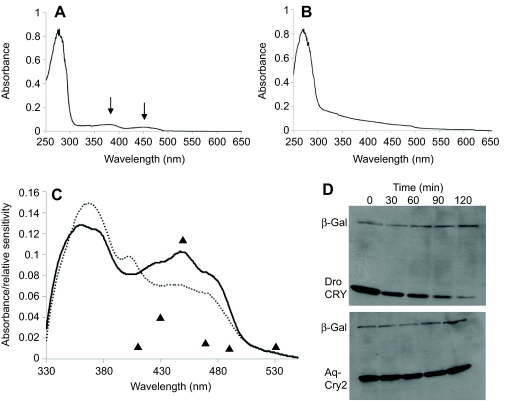
Fig. 5.
Spectroscopic properties and proteolytic activity of A. queenslandica cryptochromes. (A,B) Absorption spectra of purified Aq-Cry2 (A) and Aq-Cry1 (B). Arrows in A point to peaks, indicating purification of the protein along with stoichiometric flavin. The lack of measurable absorption in the 350–350 nm range in B suggests that Aq-Cry1 (AqCRY1-V-5/HisA) did not contain substantial flavin after purification. MBP-AqCry1 showed the same result (data not shown). In contrast, the ratio of absorbance at 280 nm of apoprotein to the 450 nm flavin adenine dinucleotide absorption peaks suggests that the recombinant MBP-AqCry2 contained stoichiometric flavin. (C) Photoreduction of Aq-Cry2 by blue light. Solid line: spectrum of Aq-Cry2 purified under yellow light. Dashed line: after exposure to UV light (366 nm at 2 mW cm–2 for 15 min). Aq-Cry2 showed the typical type-1 cryptochrome reduction pattern, with an increase of the 366 nm peak and the formation of a new peak at 405 nm and concomitant decrease of the 450 nm peak. Triangles indicate the behavioral response in living larvae (Leys et al., 2002); the minor behavioral response to 600 nm light not shown (units for behavioral response are arbitrary). (D) Light-induced proteolysis of cryptochromes. Although Aq-Cry2 displays spectroscopic properties similar to animal type-1 cryptochromes, it does not undergo photo-induced proteolysis. Drosophila CRY (Dro CRY) and Aq-Cry2 were transfected into S2 cells alongside with beta-galactosidase (β-Gal) as an internal control, and tested for light-induced proteolysis after 36 h. Although the Drosophila CRY protein was degraded via light-induced proteolysis (upper panel), levels of Aq-Cry2 remained high even after 2 h of light activation (lower panel).