Fig. 1.
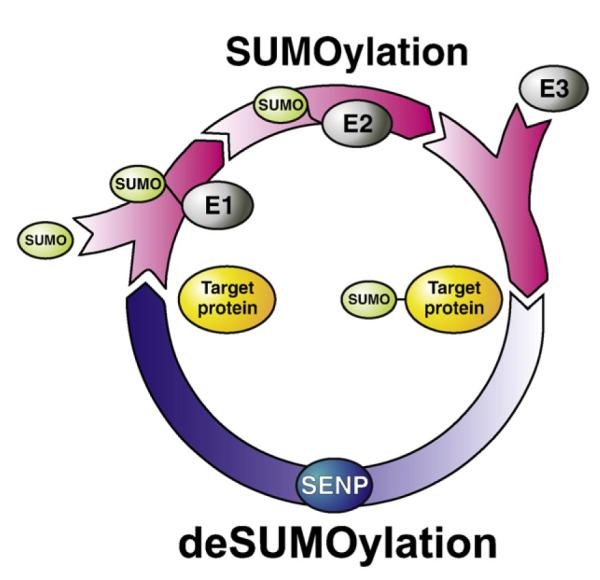
SUMO conjugation. SUMO proteins are produced as immature pre-proteins that are first cleaved C-terminally by the SENP proteins to allow their conjugation to substrate proteins (not shown). SUMO proteins are then ‘activated’ for conjugation by formation of a thioester bond between the C-terminus of SUMO and the active site cysteine of the E1 complex in an ATP-dependent process. SUMO is then passed to the active site cysteine of the sole reported SUMO-specific E2 enzyme, Ubc9. Ubc9, either alone or in conjunction with one of a number of reported E3 enzymes, then conjugates SUMO to the lysine residue in the substrate protein, altering the properties of the substrate. SUMOylation can subsequently be reversed, again by the activity of the SENP enzyme.
