Table 2. Enantioselective α-Trifluoromethylation: Aldehyde Scope.
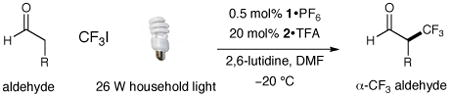 | |||||
|---|---|---|---|---|---|
| entry | producta | yield,b eec | entry | producta | yield,b eec |
| 1 |
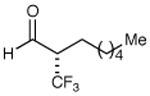
|
79% yield 99% ee | 7 |
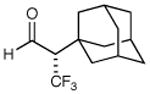
|
73% yieldd 90% ee |
| 2 |
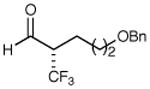
|
72% yield 95% ee | 8 |
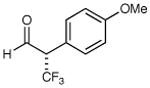
|
61% yield 93% ee |
| 3 |
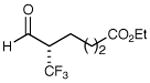
|
86% yield 97% ee | 9 |
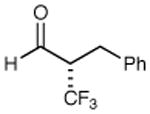
|
75% yield 97% ee |
| 4 |
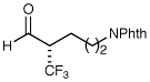
|
78% yield 98% ee | 10 |
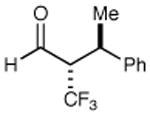
|
68% yield >20:1 dr 99% ee |
| 5 |
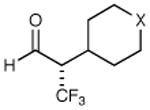
|
X = CH2 70% yield 99% ee | 11 |
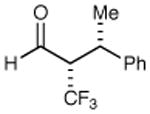
|
62% yield >20:1 dr 99% ee |
| 6 | X = NBoc 70% yield 98% ee | ||||
Stereochemistry assigned by chemical correlation or by analogy.
Isolated yields of the corresponding alcohol.
Enantiomeric excess determined by chiral SFC or HPLC analysis.
Using catalyst 11; ref 20.
