Figure 2. Proposed mechanism for the direct trifluoromethylation of aryl C–H bonds via photoredox catalysis.
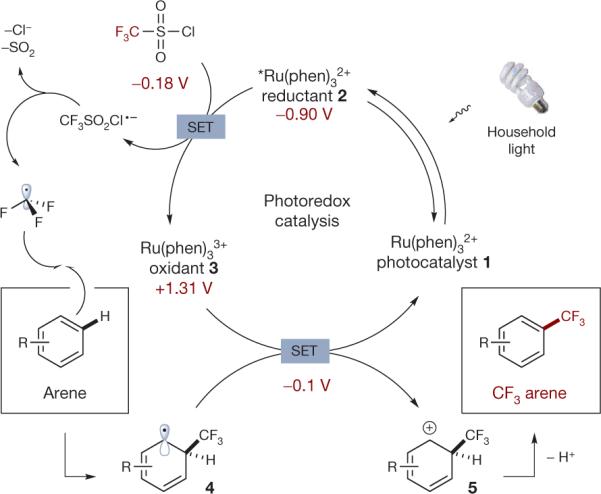
The photoredox catalytic cycle is initiated via excitation of photocatalyst 1 to excited state 2 with a household light bulb. Subsequent reduction of triflyl chloride by reductant 2 (via single electron transfer, that is, SET) provides oxidant 3 along with the radical anion of triflyl chloride. This high energy species spontaneously collapses to form CF3 radical (top left), which selectively combines with aromatic systems enabling direct CF3 substitution. Catalyst 3-promoted oxidation of the ensuing radical 4 completes the catalytic cycle and provides cyclohexadienyl cation 5, whose facile deprotonation provides the desired CF3 arene. This mechanism is consistent with measured redox values (shown below respective compounds, in maroon).
