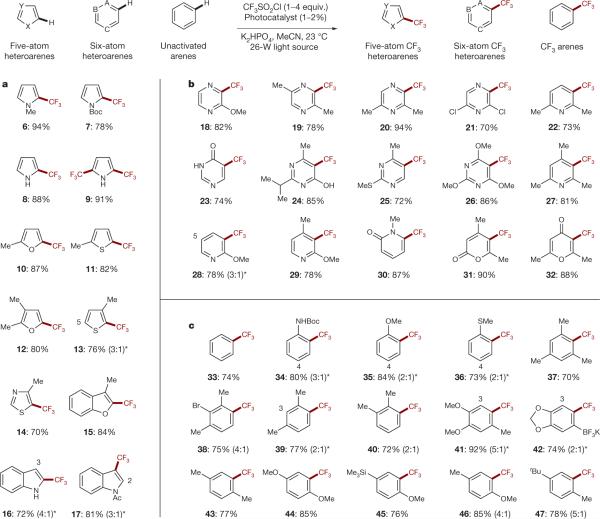
Figure 3. Radical trifluoromethylation of five- and six-membered heteroarenes and C–H arenes via photoredox catalysis.
Aromatic and heteroaromatic systems with varying stereoelectronics are efficiently trifluoromethylated under these standard conditions (top, generalized reaction). Substrate scope includes electron-rich five-atom heteroarenes (a), electron-deficient six-atom heteroarenes (b), and unactivated arenes (c). Isolated yields are indicated below each entry (19F NMR yields for volatile compounds). See Supplementary Information for experimental details. Abbreviations: X, Y, A, B, C represent either C, N, O, or S; MeCN, acetonitrile; Me, methyl; Boc, tert-butoxycarbonyl; Ac, acyl; tBu, tert-butyl. *The minor regioisomeric position is labelled with the respective carbon atom number.